Local Regulation of Fat Metabolism in Peripheral Nerves
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2002/0152483 A1 Reue Et Al
US 2002O1524.83A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0152483 A1 Reue et al. (43) Pub. Date: Oct. 17, 2002 (54) NOVEL GENE ASSOCATED WITH Publication Classification REGULATION OF ADPOSITY AND INSULIN RESPONSE (51) Int. Cl." ............................ A01K 67/00; C12O 1/68; C12P 19/34 (75) Inventors: Karen Reue, Torrance, CA (US); (52) U.S. Cl. .................................. 800/9; 435/6; 435/91.2 Miklos Peterfy, Los Angeles, CA (US) (57) ABSTRACT Correspondence Address: This invention pertains to the identification and isolation of LAW OFFICES OF JONATHAN ALAN QUINE a gene implicated in the fatty liver dystrophy (fld) pheno PO BOX 458 type. Mouse and human forms of the novel gene, designated ALAMEDA, CA 94501 herein as Lpin1/LPIN1 (mouse and human genes, respec tively), are identified. This invention additionally provides (73) Assignee: The Regents of the University of Cali methods of Screening for agents that alter adipose tissue fornia development. The methods involve contacting a cell con taining a Lpin1 gene With a test agent, and detecting a (21) Appl. No.: 10/028,056 change in the expression or activity of a Lpin1 gene product, (22) Filed: Dec. 19, 2001 where a difference in the expression or activity of Lpin1 in the contacted cell indicates that the agent alters or is likely Related U.S. Application Data to alter adipose tissue development. Also provided are methods of identifying Lpin1 mutations, and methods of (60) Provisional application No. 60/257,772, filed on Dec. mitigating Symptoms of lipodystrophy, obesity, diabetes, 22, 2000. atherOSclerosis and related pathologies. -

Inherited Neuropathies
407 Inherited Neuropathies Vera Fridman, MD1 M. M. Reilly, MD, FRCP, FRCPI2 1 Department of Neurology, Neuromuscular Diagnostic Center, Address for correspondence Vera Fridman, MD, Neuromuscular Massachusetts General Hospital, Boston, Massachusetts Diagnostic Center, Massachusetts General Hospital, Boston, 2 MRC Centre for Neuromuscular Diseases, UCL Institute of Neurology Massachusetts, 165 Cambridge St. Boston, MA 02114 and The National Hospital for Neurology and Neurosurgery, Queen (e-mail: [email protected]). Square, London, United Kingdom Semin Neurol 2015;35:407–423. Abstract Hereditary neuropathies (HNs) are among the most common inherited neurologic Keywords disorders and are diverse both clinically and genetically. Recent genetic advances have ► hereditary contributed to a rapid expansion of identifiable causes of HN and have broadened the neuropathy phenotypic spectrum associated with many of the causative mutations. The underlying ► Charcot-Marie-Tooth molecular pathways of disease have also been better delineated, leading to the promise disease for potential treatments. This chapter reviews the clinical and biological aspects of the ► hereditary sensory common causes of HN and addresses the challenges of approaching the diagnostic and motor workup of these conditions in a rapidly evolving genetic landscape. neuropathy ► hereditary sensory and autonomic neuropathy Hereditary neuropathies (HN) are among the most common Select forms of HN also involve cranial nerves and respiratory inherited neurologic diseases, with a prevalence of 1 in 2,500 function. Nevertheless, in the majority of patients with HN individuals.1,2 They encompass a clinically heterogeneous set there is no shortening of life expectancy. of disorders and vary greatly in severity, spanning a spectrum Historically, hereditary neuropathies have been classified from mildly symptomatic forms to those resulting in severe based on the primary site of nerve pathology (myelin vs. -

Target-Derived Neurotrophins Coordinate Transcription and Transport of Bclw to Prevent Axonal Degeneration
The Journal of Neuroscience, March 20, 2013 • 33(12):5195–5207 • 5195 Neurobiology of Disease Target-Derived Neurotrophins Coordinate Transcription and Transport of Bclw to Prevent Axonal Degeneration Katharina E. Cosker,1,2,3 Maria F. Pazyra-Murphy,1,2,3 Sara J. Fenstermacher,1,2,3 and Rosalind A. Segal1,2,3 1Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115, and Departments of 2Cancer Biology and 3Pediatric Oncology, Dana- Farber Cancer Institute, Boston, Massachusetts 02215 Establishment of neuronal circuitry depends on both formation and refinement of neural connections. During this process, target- derived neurotrophins regulate both transcription and translation to enable selective axon survival or elimination. However, it is not known whether retrograde signaling pathways that control transcription are coordinated with neurotrophin-regulated actions that transpire in the axon. Here we report that target-derived neurotrophins coordinate transcription of the antiapoptotic gene bclw with transport of bclw mRNA to the axon, and thereby prevent axonal degeneration in rat and mouse sensory neurons. We show that neurotrophin stimulation of nerve terminals elicits new bclw transcripts that are immediately transported to the axons and translated into protein. Bclw interacts with Bax and suppresses the caspase6 apoptotic cascade that fosters axonal degeneration. The scope of bclw regulation at the levels of transcription, transport, and translation provides a mechanism whereby sustained neurotrophin stimulation -

(12) Patent Application Publication (10) Pub. No.: US 2007/0254315 A1 Cox Et Al
US 20070254315A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0254315 A1 Cox et al. (43) Pub. Date: Nov. 1, 2007 (54) SCREENING FOR NEUROTOXIC AMINO (60) Provisional application No. 60/494.686, filed on Aug. ACID ASSOCATED WITH NEUROLOGICAL 12, 2003. DSORDERS Publication Classification (75) Inventors: Paul A. Cox, Provo, UT (US); Sandra A. Banack, Fullerton, CA (US); Susan (51) Int. Cl. J. Murch, Cambridge (CA) GOIN 33/566 (2006.01) GOIN 33/567 (2006.01) Correspondence Address: (52) U.S. Cl. ............................................................ 435/721 PILLSBURY WINTHROP SHAW PITTMAN LLP (57) ABSTRACT ATTENTION: DOCKETING DEPARTMENT Methods for screening for neurological disorders are dis P.O BOX 105OO closed. Specifically, methods are disclosed for screening for McLean, VA 22102 (US) neurological disorders in a Subject by analyzing a tissue sample obtained from the subject for the presence of (73) Assignee: THE INSTITUTE FOR ETHNO elevated levels of neurotoxic amino acids or neurotoxic MEDICINE, Provo, UT derivatives thereof associated with neurological disorders. In particular, methods are disclosed for diagnosing a neu (21) Appl. No.: 11/760,668 rological disorder in a subject, or predicting the likelihood of developing a neurological disorder in a Subject, by deter (22) Filed: Jun. 8, 2007 mining the levels of B-N-methylamino-L-alanine (BMAA) Related U.S. Application Data in a tissue sample obtained from the subject. Methods for screening for environmental factors associated with neuro (63) Continuation of application No. 10/731,411, filed on logical disorders are disclosed. Methods for inhibiting, treat Dec. 8, 2003, now Pat. No. 7,256,002. -

A Case Report on Charcot-Marie-Tooth Disease with a Novel Periaxin Gene Mutation
Open Access Case Report DOI: 10.7759/cureus.5111 A Case Report on Charcot-Marie-Tooth Disease with a Novel Periaxin Gene Mutation Sorabh Datta 1 , Saurabh Kataria 1 , Raghav Govindarajan 1 1. Neurology, University of Missouri, Columbia, USA Corresponding author: Sorabh Datta, [email protected] Abstract Charcot-Marie-Tooth (CMT) disease is one of the most common primary hereditary neuropathies causing peripheral neuropathies. More than 60 different gene mutations are causing this disease. The PRX gene codes for Periaxin proteins that are expressed by Schwann cells and are necessary for the formation and maintenance of myelination of peripheral nerves. Dejerine-Sottas neuropathy and Charcot-Marie-Tooth type 4F (CMT4F) are the two different clinical phenotypes observed in association with PRX gene mutation. This article describes a case of an elderly male with a novel mutation involving the PRX gene. Categories: Genetics, Internal Medicine, Neurology Keywords: neurology, sensorimotor neuropathy, congenital, gene expression, genetic mutation, protein, pes cavus, demyelinating diseases, charcot-marie-tooth, autosomal recessive disorder Introduction As per the Dyck classification in the year 1970, primary hereditary neuropathies are divided into hereditary motor sensory neuropathy (HMSN) and hereditary sensory autonomic neuropathy (HSAN) [1]. Charcot- Marie-Tooth (CMT) disease is a type of HMSN with an estimated prevalence of 1 in 2,500 [2]. CMT can follow autosomal recessive (ARCMT), X-linked recessive, and also an autosomal dominant pattern. CMT type 4 is a rapidly increasing ARCMT disease form in HMSN, although CMT type 1 and 2 still account for the most substantial proportion of the patient population [3]. CMT4F is a severe, demyelinating subtype of CMT type 4 and is characterized by childhood onset of slowly progressing weakness in the distal muscles associated with atrophy. -

Peripheral Neuropathy in Complex Inherited Diseases: an Approach To
PERIPHERAL NEUROPATHY IN COMPLEX INHERITED DISEASES: AN APPROACH TO DIAGNOSIS Rossor AM1*, Carr AS1*, Devine H1, Chandrashekar H2, Pelayo-Negro AL1, Pareyson D3, Shy ME4, Scherer SS5, Reilly MM1. 1. MRC Centre for Neuromuscular Diseases, UCL Institute of Neurology and National Hospital for Neurology and Neurosurgery, London, WC1N 3BG, UK. 2. Lysholm Department of Neuroradiology, National Hospital for Neurology and Neurosurgery, London, WC1N 3BG, UK. 3. Unit of Neurological Rare Diseases of Adulthood, Carlo Besta Neurological Institute IRCCS Foundation, Milan, Italy. 4. Department of Neurology, University of Iowa, 200 Hawkins Drive, Iowa City, IA 52242, USA 5. Department of Neurology, University of Pennsylvania, Philadelphia, PA 19014, USA. * These authors contributed equally to this work Corresponding author: Mary M Reilly Address: MRC Centre for Neuromuscular Diseases, 8-11 Queen Square, London, WC1N 3BG, UK. Email: [email protected] Telephone: 0044 (0) 203 456 7890 Word count: 4825 ABSTRACT Peripheral neuropathy is a common finding in patients with complex inherited neurological diseases and may be subclinical or a major component of the phenotype. This review aims to provide a clinical approach to the diagnosis of this complex group of patients by addressing key questions including the predominant neurological syndrome associated with the neuropathy e.g. spasticity, the type of neuropathy, and the other neurological and non- neurological features of the syndrome. Priority is given to the diagnosis of treatable conditions. Using this approach, we associated neuropathy with one of three major syndromic categories - 1) ataxia, 2) spasticity, and 3) global neurodevelopmental impairment. Syndromes that do not fall easily into one of these three categories can be grouped according to the predominant system involved in addition to the neuropathy e.g. -
Case Vignettes
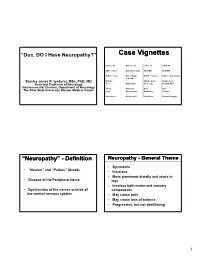
“Doc, DO I Have Neuropathy?” Case Vignettes Susan, 50 Gwenn, 32 John, 52 Sally, 42 DM – 12 yrs Hypothyroidism No PMH No PMH B/N/T – 2 yrs N/T at night B/N/T – 6 days B/N/T – few months 3 months Stanley Jones P. Iyadurai, MSc, PhD, MD Hands, Hands, Arms Hands, Feet Assistant Professor of Neurology Feet Right hand Feet, Legs Burning Pain Neuromuscular Division, Department of Neurology Arms, Difficulty Pain Pain The Ohio State University Wexner Medical Center Legs Opening jars Breathing Redness Imbalance Normal gait Imbalance Normal Strength “Neuropathy” - Definition Neuropathy - General Theme • Symmetric • “Neuron” and “Pathos” (Greek) • Insidious • More prominent distally and starts in • Disease of the Peripheral Nerve legs • Involves both motor and sensory • Dysfunction of the nerves outside of components the central nervous system • May cause pain • May cause loss of balance • Progressive, but not debilitating 1 Not all that tingles is Classification - Functional neuropathy ‘numbness and tingling’ • Motor – affects the motor nerves Weakness • not all numbness and tingling is • Sensory peripheral nerve ‒ Pain • RLS ‒ Small Fiber ‒ Large Fiber • edema ‒ Large and Small Fiber • deconditioning ‒ Neuronopathy (ganglionopathy) • central nervous system • Autonomic • Sweating Changes, Blood Pressure Changes • nerve root [‘sciatica’] Classification – Time-course Classification – Time-course • Acute Immune-mediated • Congenital/Hereditary • Infantile Weakness • Childhood-onset • Acquired • Relapsing ‒ Reversible? • Hereditary ‒ Demyelinating? -

Ataxia with Loss of Purkinje Cells in a Mouse Model for Refsum Disease
Ataxia with loss of Purkinje cells in a mouse model for Refsum disease Sacha Ferdinandussea,1,2, Anna W. M. Zomerb,1, Jasper C. Komena, Christina E. van den Brinkb, Melissa Thanosa, Frank P. T. Hamersc, Ronald J. A. Wandersa,d, Paul T. van der Saagb, Bwee Tien Poll-Thed, and Pedro Britesa Academic Medical Center, Departments of aClinical Chemistry (Laboratory of Genetic Metabolic Diseases) and dPediatrics, Emma’s Children Hospital, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; bHubrecht Institute, Royal Netherlands Academy of Arts and Sciences 3584 CT Utrecht, The Netherlands; and cRehabilitation Hospital ‘‘De Hoogstraat’’ Rudolf Magnus Institute of Neuroscience, 3584 CG Utrecht, The Netherlands Edited by P. Borst, The Netherlands Cancer Institute, Amsterdam, The Netherlands, and approved October 3, 2008 (received for review June 23, 2008) Refsum disease is caused by a deficiency of phytanoyl-CoA hy- Clinically, Refsum disease is characterized by cerebellar droxylase (PHYH), the first enzyme of the peroxisomal ␣-oxidation ataxia, polyneuropathy, and progressive retinitis pigmentosa, system, resulting in the accumulation of the branched-chain fatty culminating in blindness, (1, 3). The age of onset of the symptoms acid phytanic acid. The main clinical symptoms are polyneuropathy, can vary from early childhood to the third or fourth decade of cerebellar ataxia, and retinitis pigmentosa. To study the patho- life. No treatment is available for patients with Refsum disease, genesis of Refsum disease, we generated and characterized a Phyh but they benefit from a low phytanic acid diet. Phytanic acid is knockout mouse. We studied the pathological effects of phytanic derived from dietary sources only, specifically from the chloro- acid accumulation in Phyh؊/؊ mice fed a diet supplemented with phyll component phytol. -

ICD9 & ICD10 Neuromuscular Codes
ICD-9-CM and ICD-10-CM NEUROMUSCULAR DIAGNOSIS CODES ICD-9-CM ICD-10-CM Focal Neuropathy Mononeuropathy G56.00 Carpal tunnel syndrome, unspecified Carpal tunnel syndrome 354.00 G56.00 upper limb Other lesions of median nerve, Other median nerve lesion 354.10 G56.10 unspecified upper limb Lesion of ulnar nerve, unspecified Lesion of ulnar nerve 354.20 G56.20 upper limb Lesion of radial nerve, unspecified Lesion of radial nerve 354.30 G56.30 upper limb Lesion of sciatic nerve, unspecified Sciatic nerve lesion (Piriformis syndrome) 355.00 G57.00 lower limb Meralgia paresthetica, unspecified Meralgia paresthetica 355.10 G57.10 lower limb Lesion of lateral popiteal nerve, Peroneal nerve (lesion of lateral popiteal nerve) 355.30 G57.30 unspecified lower limb Tarsal tunnel syndrome, unspecified Tarsal tunnel syndrome 355.50 G57.50 lower limb Plexus Brachial plexus lesion 353.00 Brachial plexus disorders G54.0 Brachial neuralgia (or radiculitis NOS) 723.40 Radiculopathy, cervical region M54.12 Radiculopathy, cervicothoracic region M54.13 Thoracic outlet syndrome (Thoracic root Thoracic root disorders, not elsewhere 353.00 G54.3 lesions, not elsewhere classified) classified Lumbosacral plexus lesion 353.10 Lumbosacral plexus disorders G54.1 Neuralgic amyotrophy 353.50 Neuralgic amyotrophy G54.5 Root Cervical radiculopathy (Intervertebral disc Cervical disc disorder with myelopathy, 722.71 M50.00 disorder with myelopathy, cervical region) unspecified cervical region Lumbosacral root lesions (Degeneration of Other intervertebral disc degeneration, -

(12) Patent Application Publication (10) Pub. No.: US 2008/0057509 A1 Lupski Et Al
US 2008005.7509A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0057509 A1 Lupski et al. (43) Pub. Date: Mar. 6, 2008 (54) DEFECTS IN PERIAXIN ASSOCIATED WITH (60) Provisional application No. 60/255.217, filed on Dec. MYELINOPATHIES 13, 2000. (76) Inventors: James R. Lupski, Houston, TX (US); Publication Classification Cornelius F. Boerkoel III, Houston, TX (US); Hiroshi Takashima, Houston, (51) Int. Cl. TX (US) CI2O I/68 (2006.01) (52) U.S. Cl. .................................................................. 435/6 Correspondence Address: FULBRIGHT & JAWORSKI, LLP 1301 MCKNNEY SUTE 51OO (57) ABSTRACT HOUSTON, TX 77010-3095 (US) (21) Appl. No.: 11/838,500 The present invention relates to defects in periaxin (PRX) associated with myelinopathies, including Charcot-Marie (22) Filed: Aug. 14, 2007 Tooth syndrome and/or Deerine-Sottas syndrome. Unre lated individuals having a myelinopathy from Dejerine Related U.S. Application Data Sottas syndrome have recessive PRX mutations. The PRX locus maps to a region associated with a severe autosomal (63) Continuation of application No. 10/021,955, filed on recessive demyelinating neuropathy and is also syntenic to Dec. 13, 2001, now Pat. No. 7,273,698. the Prx location on murine chromosome 7. PN-44. v Fifi is Gf * is f. f i? it it it it i? it is 86 if f : Gift it if is f. 6 it is a a is 8 is a B g g it a fit i AN AIK hetero 45A homoliSA homoASA CSX 7 Six Taar a via a unania t fi : Yi 3 y Clas?.(CENELY I II. Ex. -

1 First Presentation of LPIN1 Acute Rhabdomyolysis in Adolescence and Adulthood
Manuscript File Click here to view linked References 1 First presentation of LPIN1 acute rhabdomyolysis in adolescence and adulthood 2 3 Chiara Pizzamiglioa, Nayana Lahirib, Niranjanan Nirmalananthanc, Bhrigu Soodd, Subash 4 Somalankad, Philip Ostrowskie, Rahul Phadkef, Dominic Gerard O’Donovang, Francesco 5 Muntonih, Rosaline Quinlivana 6 7 a. MRC Centre for Neuromuscular Diseases, UCL Institute of Neurology and National Hospital for 8 Neurology and Neurosurgery, Queen Square, London, United Kingdom 9 10 b. Clinical Genetics Department, St George's University Hospitals NHS Foundation Trust, London, 11 United Kingdom 12 13 c. Departments of Neurology and Neuroradiology, Atkinson Morley Regional Neurosciences 14 Centre, St George's Hospital, London, United Kingdom 15 16 d. South West Thames Renal & Transplantation Unit and South West Thames Institute for Renal 17 Research, Saint Helier Hospital, Carshalton, Surrey, United Kingdom 18 19 e. South West Thames Regional Genetics Service, St George's University NHS Foundation Trust, 20 London, United Kingdom 21 22 f. Division of Neuropathology, Dubowitz Neuromuscular Centre, UCL Great Ormond Street 23 Hospital for Children, United Kingdom; Division of Neuropathology, National Hospital for 24 Neurology and Neurosurgery, Queen Square, London, United Kingdom 25 1 26 g. Neuropathology, Department of Histopathology, Addenbrooke’s Hospital, Cambridge University 27 Hospitals NHS Foundation Trust, Cambridge, United Kingdom 28 29 h. Paediatric Neurology, Dubowitz Neuromuscular Centre, UCL Institute of Child Health and Great 30 Ormond Street Hospital for Children, London, United Kingdom 31 32 Corresponding author: Chiara Pizzamiglio ([email protected]) 33 34 Abstract: LPIN1 mutations are a known common cause of autosomal recessive, recurrent and life- 35 threatening acute rhabdomyolysis of childhood-onset. -

Prediction of Tissue-Specific Cis-Regulatory Sequences: Application to the Ascidian Ciona Intestinalis and the Anterior Neurectoderm Maximilian Häussler
Prediction of tissue-specific cis-regulatory sequences: application to the ascidian Ciona intestinalis and the anterior neurectoderm Maximilian Häussler To cite this version: Maximilian Häussler. Prediction of tissue-specific cis-regulatory sequences: application to the ascidian Ciona intestinalis and the anterior neurectoderm. Cellular Biology. Université Paris Sud - Paris XI, 2009. English. tel-00413501 HAL Id: tel-00413501 https://tel.archives-ouvertes.fr/tel-00413501 Submitted on 4 Sep 2009 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Université Paris XI Discipline Biologie Cellulaire et Moléculaire École doctorale Gènes, Génomes, Cellules Thèse pour obtenir le grade de Docteur de l'Université Paris XI Soutenance prévu le 15. Juillet 2009 par Maximilian Häussler Prédiction des séquences cis-regulatrices tissu-spéci- fiques: application à l'ascidie Ciona intestinalis et au neurectoderme antérieur Prediction of tissue-specific cis-regulatory sequences: application to the ascidian Ciona intestinalis and the anterior neurectoderm Jury President M. Pierre Capy Rapporteurs: M. Nicolas Pollet M. Sebastian Shimeld Examinateur: M. Elia Stupka Directeur de thèse: M. Jean-Stéphane Joly This thesis can be downloaded from http://hal.archives-ouvertes.fr as a PDF file Summary The detection and annotation of cis-regulatory sequences is a difficult problem.