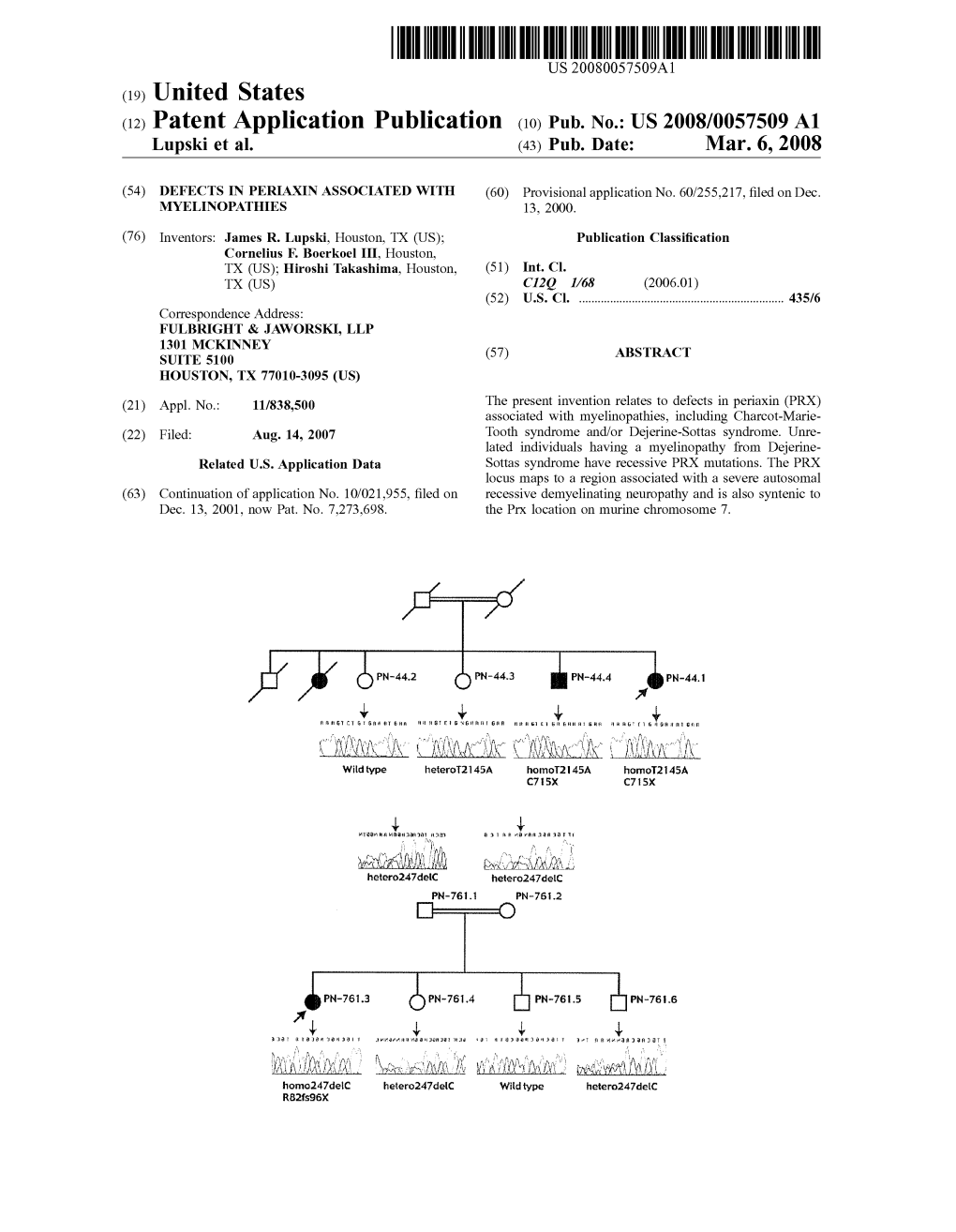
(12) Patent Application Publication (10) Pub. No.: US 2008/0057509 A1 Lupski Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inherited Neuropathies
407 Inherited Neuropathies Vera Fridman, MD1 M. M. Reilly, MD, FRCP, FRCPI2 1 Department of Neurology, Neuromuscular Diagnostic Center, Address for correspondence Vera Fridman, MD, Neuromuscular Massachusetts General Hospital, Boston, Massachusetts Diagnostic Center, Massachusetts General Hospital, Boston, 2 MRC Centre for Neuromuscular Diseases, UCL Institute of Neurology Massachusetts, 165 Cambridge St. Boston, MA 02114 and The National Hospital for Neurology and Neurosurgery, Queen (e-mail: [email protected]). Square, London, United Kingdom Semin Neurol 2015;35:407–423. Abstract Hereditary neuropathies (HNs) are among the most common inherited neurologic Keywords disorders and are diverse both clinically and genetically. Recent genetic advances have ► hereditary contributed to a rapid expansion of identifiable causes of HN and have broadened the neuropathy phenotypic spectrum associated with many of the causative mutations. The underlying ► Charcot-Marie-Tooth molecular pathways of disease have also been better delineated, leading to the promise disease for potential treatments. This chapter reviews the clinical and biological aspects of the ► hereditary sensory common causes of HN and addresses the challenges of approaching the diagnostic and motor workup of these conditions in a rapidly evolving genetic landscape. neuropathy ► hereditary sensory and autonomic neuropathy Hereditary neuropathies (HN) are among the most common Select forms of HN also involve cranial nerves and respiratory inherited neurologic diseases, with a prevalence of 1 in 2,500 function. Nevertheless, in the majority of patients with HN individuals.1,2 They encompass a clinically heterogeneous set there is no shortening of life expectancy. of disorders and vary greatly in severity, spanning a spectrum Historically, hereditary neuropathies have been classified from mildly symptomatic forms to those resulting in severe based on the primary site of nerve pathology (myelin vs. -

A Case Report on Charcot-Marie-Tooth Disease with a Novel Periaxin Gene Mutation
Open Access Case Report DOI: 10.7759/cureus.5111 A Case Report on Charcot-Marie-Tooth Disease with a Novel Periaxin Gene Mutation Sorabh Datta 1 , Saurabh Kataria 1 , Raghav Govindarajan 1 1. Neurology, University of Missouri, Columbia, USA Corresponding author: Sorabh Datta, [email protected] Abstract Charcot-Marie-Tooth (CMT) disease is one of the most common primary hereditary neuropathies causing peripheral neuropathies. More than 60 different gene mutations are causing this disease. The PRX gene codes for Periaxin proteins that are expressed by Schwann cells and are necessary for the formation and maintenance of myelination of peripheral nerves. Dejerine-Sottas neuropathy and Charcot-Marie-Tooth type 4F (CMT4F) are the two different clinical phenotypes observed in association with PRX gene mutation. This article describes a case of an elderly male with a novel mutation involving the PRX gene. Categories: Genetics, Internal Medicine, Neurology Keywords: neurology, sensorimotor neuropathy, congenital, gene expression, genetic mutation, protein, pes cavus, demyelinating diseases, charcot-marie-tooth, autosomal recessive disorder Introduction As per the Dyck classification in the year 1970, primary hereditary neuropathies are divided into hereditary motor sensory neuropathy (HMSN) and hereditary sensory autonomic neuropathy (HSAN) [1]. Charcot- Marie-Tooth (CMT) disease is a type of HMSN with an estimated prevalence of 1 in 2,500 [2]. CMT can follow autosomal recessive (ARCMT), X-linked recessive, and also an autosomal dominant pattern. CMT type 4 is a rapidly increasing ARCMT disease form in HMSN, although CMT type 1 and 2 still account for the most substantial proportion of the patient population [3]. CMT4F is a severe, demyelinating subtype of CMT type 4 and is characterized by childhood onset of slowly progressing weakness in the distal muscles associated with atrophy. -

Prediction of Tissue-Specific Cis-Regulatory Sequences: Application to the Ascidian Ciona Intestinalis and the Anterior Neurectoderm Maximilian Häussler
Prediction of tissue-specific cis-regulatory sequences: application to the ascidian Ciona intestinalis and the anterior neurectoderm Maximilian Häussler To cite this version: Maximilian Häussler. Prediction of tissue-specific cis-regulatory sequences: application to the ascidian Ciona intestinalis and the anterior neurectoderm. Cellular Biology. Université Paris Sud - Paris XI, 2009. English. tel-00413501 HAL Id: tel-00413501 https://tel.archives-ouvertes.fr/tel-00413501 Submitted on 4 Sep 2009 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Université Paris XI Discipline Biologie Cellulaire et Moléculaire École doctorale Gènes, Génomes, Cellules Thèse pour obtenir le grade de Docteur de l'Université Paris XI Soutenance prévu le 15. Juillet 2009 par Maximilian Häussler Prédiction des séquences cis-regulatrices tissu-spéci- fiques: application à l'ascidie Ciona intestinalis et au neurectoderme antérieur Prediction of tissue-specific cis-regulatory sequences: application to the ascidian Ciona intestinalis and the anterior neurectoderm Jury President M. Pierre Capy Rapporteurs: M. Nicolas Pollet M. Sebastian Shimeld Examinateur: M. Elia Stupka Directeur de thèse: M. Jean-Stéphane Joly This thesis can be downloaded from http://hal.archives-ouvertes.fr as a PDF file Summary The detection and annotation of cis-regulatory sequences is a difficult problem. -

In This Table Protein Name, Uniprot Code, Gene Name P-Value
Supplementary Table S1: In this table protein name, uniprot code, gene name p-value and Fold change (FC) for each comparison are shown, for 299 of the 301 significantly regulated proteins found in both comparisons (p-value<0.01, fold change (FC) >+/-0.37) ALS versus control and FTLD-U versus control. Two uncharacterized proteins have been excluded from this list Protein name Uniprot Gene name p value FC FTLD-U p value FC ALS FTLD-U ALS Cytochrome b-c1 complex P14927 UQCRB 1.534E-03 -1.591E+00 6.005E-04 -1.639E+00 subunit 7 NADH dehydrogenase O95182 NDUFA7 4.127E-04 -9.471E-01 3.467E-05 -1.643E+00 [ubiquinone] 1 alpha subcomplex subunit 7 NADH dehydrogenase O43678 NDUFA2 3.230E-04 -9.145E-01 2.113E-04 -1.450E+00 [ubiquinone] 1 alpha subcomplex subunit 2 NADH dehydrogenase O43920 NDUFS5 1.769E-04 -8.829E-01 3.235E-05 -1.007E+00 [ubiquinone] iron-sulfur protein 5 ARF GTPase-activating A0A0C4DGN6 GIT1 1.306E-03 -8.810E-01 1.115E-03 -7.228E-01 protein GIT1 Methylglutaconyl-CoA Q13825 AUH 6.097E-04 -7.666E-01 5.619E-06 -1.178E+00 hydratase, mitochondrial ADP/ATP translocase 1 P12235 SLC25A4 6.068E-03 -6.095E-01 3.595E-04 -1.011E+00 MIC J3QTA6 CHCHD6 1.090E-04 -5.913E-01 2.124E-03 -5.948E-01 MIC J3QTA6 CHCHD6 1.090E-04 -5.913E-01 2.124E-03 -5.948E-01 Protein kinase C and casein Q9BY11 PACSIN1 3.837E-03 -5.863E-01 3.680E-06 -1.824E+00 kinase substrate in neurons protein 1 Tubulin polymerization- O94811 TPPP 6.466E-03 -5.755E-01 6.943E-06 -1.169E+00 promoting protein MIC C9JRZ6 CHCHD3 2.912E-02 -6.187E-01 2.195E-03 -9.781E-01 Mitochondrial 2- -

Peroxiredoxins: Guardians Against Oxidative Stress and Modulators of Peroxide Signaling
Peroxiredoxins: Guardians Against Oxidative Stress and Modulators of Peroxide Signaling Perkins, A., Nelson, K. J., Parsonage, D., Poole, L. B., & Karplus, P. A. (2015). Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling. Trends in Biochemical Sciences, 40(8), 435-445. doi:10.1016/j.tibs.2015.05.001 10.1016/j.tibs.2015.05.001 Elsevier Accepted Manuscript http://cdss.library.oregonstate.edu/sa-termsofuse Revised Manuscript clean Click here to download Manuscript: Peroxiredoxin-TiBS-revised-4-25-15-clean.docx 1 2 3 4 5 6 7 8 9 Peroxiredoxins: Guardians Against Oxidative Stress and Modulators of 10 11 12 Peroxide Signaling 13 14 15 16 17 18 19 1 2 2 2 20 Arden Perkins, Kimberly J. Nelson, Derek Parsonage, Leslie B. Poole * 21 22 23 and P. Andrew Karplus1* 24 25 26 27 28 29 30 31 1 Department of Biochemistry and Biophysics, Oregon State University, Corvallis, Oregon 97333 32 33 34 2 Department of Biochemistry, Wake Forest School of Medicine, Winston-Salem, North Carolina 27157 35 36 37 38 39 40 41 *To whom correspondence should be addressed: 42 43 44 L.B. Poole, ph: 336-716-6711, fax: 336-713-1283, email: [email protected] 45 46 47 P.A. Karplus, ph: 541-737-3200, fax: 541- 737-0481, email: [email protected] 48 49 50 51 52 53 54 55 Keywords: antioxidant enzyme, peroxidase, redox signaling, antioxidant defense 56 57 58 59 60 61 62 63 64 65 1 2 3 4 5 6 7 8 9 Abstract 10 11 12 13 Peroxiredoxins (Prxs) are a ubiquitous family of cysteine-dependent peroxidase enzymes that play dominant 14 15 16 roles in regulating peroxide levels within cells. -

Mouse Prx Knockout Project (CRISPR/Cas9)
https://www.alphaknockout.com Mouse Prx Knockout Project (CRISPR/Cas9) Objective: To create a Prx knockout Mouse model (C57BL/6N) by CRISPR/Cas-mediated genome engineering. Strategy summary: The Prx gene (NCBI Reference Sequence: NM_019412 ; Ensembl: ENSMUSG00000053198 ) is located on Mouse chromosome 7. 6 exons are identified, with the ATG start codon in exon 4 and the TAA stop codon in exon 6 (Transcript: ENSMUST00000108355). Exon 6 will be selected as target site. Cas9 and gRNA will be co-injected into fertilized eggs for KO Mouse production. The pups will be genotyped by PCR followed by sequencing analysis. Note: Mice homozygous for disruptions in this gene display locomotor problems as well as difficulty eating and breathing. Demyelination of peripheral nerves develops with age. Exon 6 starts from about 41.67% of the coding region. Exon 6 covers 58.56% of the coding region. The size of effective KO region: ~4964 bp. The KO region does not have any other known gene. Page 1 of 9 https://www.alphaknockout.com Overview of the Targeting Strategy Wildtype allele 5' gRNA region gRNA region 3' 1 6 Legends Exon of mouse Prx Knockout region Page 2 of 9 https://www.alphaknockout.com Overview of the Dot Plot (up) Window size: 15 bp Forward Reverse Complement Sequence 12 Note: The 2000 bp section upstream of Exon 6 is aligned with itself to determine if there are tandem repeats. Tandem repeats are found in the dot plot matrix. The gRNA site is selected outside of these tandem repeats. Overview of the Dot Plot (down) Window size: 15 bp Forward Reverse Complement Sequence 12 Note: The 2000 bp section downstream of Exon 6 is aligned with itself to determine if there are tandem repeats. -

Expression of Periaxin (PRX) Specifically in the Human
www.nature.com/scientificreports OPEN Expression of periaxin (PRX) specifcally in the human cerebrovascular system: PDZ Received: 6 November 2017 Accepted: 13 June 2018 domain-mediated strengthening Published: xx xx xxxx of endothelial barrier function Michael M. Wang1,2,3,4, Xiaojie Zhang1,2, Soo Jung Lee1,2, Snehaa Maripudi1,3, Richard F. Keep2,4,5, Allison M. Johnson4,6,7, Svetlana M. Stamatovic6,7 & Anuska V. Andjelkovic4,6,7 Regulation of cerebral endothelial cell function plays an essential role in changes in blood-brain barrier permeability. Proteins that are important for establishment of endothelial tight junctions have emerged as critical molecules, and PDZ domain containing-molecules are among the most important. We have discovered that the PDZ-domain containing protein periaxin (PRX) is expressed in human cerebral endothelial cells. Surprisingly, PRX protein is not detected in brain endothelium in other mammalian species, suggesting that it could confer human-specifc vascular properties. In endothelial cells, PRX is predominantly localized to the nucleus and not tight junctions. Transcriptome analysis shows that PRX expression suppresses, by at least 50%, a panel of infammatory markers, of which 70% are Type I interferon response genes; only four genes were signifcantly activated by PRX expression. When expressed in mouse endothelial cells, PRX strengthens barrier function, signifcantly increases transendothelial electrical resistance (~35%; p < 0.05), and reduces the permeability of a wide range of molecules. The PDZ domain of PRX is necessary and sufcient for its barrier enhancing properties, since a splice variant (S-PRX) that contains only the PDZ domain, also increases barrier function. PRX also attenuates the permeability enhancing efects of lipopolysaccharide. -

Genome-Scale Analysis of DNA Methylation in Lung Adenocarcinoma and Integration with Mrna Expression
Downloaded from genome.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press Research Genome-scale analysis of DNA methylation in lung adenocarcinoma and integration with mRNA expression Suhaida A. Selamat,1 Brian S. Chung,1 Luc Girard,2 Wei Zhang,2 Ying Zhang,3 Mihaela Campan,1 Kimberly D. Siegmund,3 Michael N. Koss,4 Jeffrey A. Hagen,5 Wan L. Lam,6 Stephen Lam,6 Adi F. Gazdar,2 and Ite A. Laird-Offringa1,7 1Department of Surgery, Department of Biochemistry and Molecular Biology, Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, California 90089-9176, USA; 2The Hamon Center for Therapeutic Oncology Research and Department of Pathology, University of Texas Southwestern Medical Center, Dallas, Texas 75390, USA; 3Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California 90089-9176, USA; 4Department of Pathology, Keck School of Medicine, University of Southern California, Los Angeles, California 90089-9176, USA; 5Department of Surgery, Keck School of Medicine, University of Southern California, Los Angeles, California 90089-9176, USA; 6BC Cancer Research Center, BC Cancer Agency, Vancouver, BC V521L3, Canada Lung cancer is the leading cause of cancer death worldwide, and adenocarcinoma is its most common histological subtype. Clinical and molecular evidence indicates that lung adenocarcinoma is a heterogeneous disease, which has important implications for treatment. Here we performed genome-scale DNA methylation profiling using the Illumina Infinium HumanMethylation27 platform on 59 matched lung adenocarcinoma/non-tumor lung pairs, with genome-scale verifi- cation on an independent set of tissues. -

A Yeast-Based Model for Hereditary Motor and Sensory Neuropathies: a Simple System for Complex, Heterogeneous Diseases
International Journal of Molecular Sciences Review A Yeast-Based Model for Hereditary Motor and Sensory Neuropathies: A Simple System for Complex, Heterogeneous Diseases Weronika Rzepnikowska 1, Joanna Kaminska 2 , Dagmara Kabzi ´nska 1 , Katarzyna Bini˛eda 1 and Andrzej Kocha ´nski 1,* 1 Neuromuscular Unit, Mossakowski Medical Research Centre Polish Academy of Sciences, 02-106 Warsaw, Poland; [email protected] (W.R.); [email protected] (D.K.); [email protected] (K.B.) 2 Institute of Biochemistry and Biophysics Polish Academy of Sciences, 02-106 Warsaw, Poland; [email protected] * Correspondence: [email protected] Received: 19 May 2020; Accepted: 15 June 2020; Published: 16 June 2020 Abstract: Charcot–Marie–Tooth (CMT) disease encompasses a group of rare disorders that are characterized by similar clinical manifestations and a high genetic heterogeneity. Such excessive diversity presents many problems. Firstly, it makes a proper genetic diagnosis much more difficult and, even when using the most advanced tools, does not guarantee that the cause of the disease will be revealed. Secondly, the molecular mechanisms underlying the observed symptoms are extremely diverse and are probably different for most of the disease subtypes. Finally, there is no possibility of finding one efficient cure for all, or even the majority of CMT diseases. Every subtype of CMT needs an individual approach backed up by its own research field. Thus, it is little surprise that our knowledge of CMT disease as a whole is selective and therapeutic approaches are limited. There is an urgent need to develop new CMT models to fill the gaps. -
A Polymorphism in the Nuclear Receptor Coactivator 7 Gene and Breast Cancer Susceptibility
ONCOLOGY LETTERS 3: 131-134, 2012 A polymorphism in the nuclear receptor coactivator 7 gene and breast cancer susceptibility JULIA SÜLLNER*, CLAUS LATTRICH*, JULIA HÄRING, REGINA GÖRSE, OLAF ORTMANN and OLIVER TREECK Department of Obstetrics and Gynecology, University Medical Center Regensburg, Regensburg, Germany Received May 9, 2011; Accepted August 24, 2011 DOI: 10.3892/ol.2011.421 Abstract. The nuclear receptor coactivator 7 (NCoA7) gene target genes (3). In addition to communicating with the tran- codes for an estrogen receptor-associated protein that plays scriptional machinery, certain coregulators are also capable a significant role in the cellular response to estrogens. Given of altering chromatin function (4). It has been suggested that that NCoA7 is expressed in the mammary gland, alterations coregulatory proteins perform virtually all of the reactions in this gene may affect breast cancer risk. In this study, we required to control enhancer-dependent gene expression. compared the genotype and allele frequencies of the missense These proteins regulate subfunctions of numerous transcrip- single nucleotide polymorphism (SNP) rs1567, located in the tion factors in addition to cell processes including translation, coding region of the NCoA7 gene and resulting in an amino energy generation and motility (5). acid exchange from asparagine to glutamine, in 305 women Nuclear receptor coactivator 7 (NCoA7) is an with sporadic breast cancer and 346 women without any ER-coactivator protein also known as ERAP140 malignancy. Statistical analysis of the observed frequencies (ER-associated protein 140) (6). Ligand-bound ERα or ERβ did not reveal a significant difference between the cancer and recruits NCoA7 to the promoter region of ER target genes control groups, nor did a comparison between histological (7). -
The Dystrobrevin-Binding Protein 1 Gene
Molecular Psychiatry (2009) 14,18–29 & 2009 Nature Publishing Group All rights reserved 1359-4184/09 $32.00 www.nature.com/mp FEATURE REVIEW The dystrobrevin-binding protein 1 gene: features and networks AY Guo1,4, J Sun1,4, BP Riley1,2, DL Thiselton1, KS Kendler1,2 and Z Zhao1,2,3 1Department of Psychiatry and Virginia Institute for Psychiatric and Behavior Genetics, Virginia Commonwealth University, Richmond, VA, USA; 2Department of Human and Molecular Genetics, Virginia Commonwealth University, Richmond, VA, USA and 3Center for the Study of Biological Complexity, Virginia Commonwealth University, Richmond, VA, USA The dystrobrevin-binding protein 1 (DTNBP1) gene has been one of the most studied and promising schizophrenia susceptibility genes since it was first reported to be associated with schizophrenia in the Irish Study of High Density Schizophrenia Families (ISHDSF). Although many studies have been performed both at the functional level and in association with psychiatric disorders, there has been no systematic review of the features of the DTNBP1 gene, protein or the relationship between function and phenotype. Using a bioinformatics approach, we identified the DTNBP1 gene in 13 vertebrate species. The comparison of these genes revealed a conserved gene structure, protein-coding sequence and dysbindin domain, but a diverse noncoding sequence. The molecular evolutionary analysis suggests the DTNBP1 gene probably originated in chordates and matured in vertebrates. No signature of recent positive selection was seen in any primate lineage. The DTNBP1 gene likely has many more alternative transcripts than the current three major isoforms annotated in the NCBI database. Our examination of risk haplotypes revealed that, although the frequency of a single nucleotide polymorphism (SNP) or haplotype might be significantly different in cases from controls, difference between major geographic populations was even larger. -

Direct Binding of the Flexible C-Terminal Segment of Periaxin to Β4 Integrin Suggests 2 a Molecular Basis for CMT4F 3 4 Arne Raasakka1, Helen Linxweiler1, Peter J
bioRxiv preprint doi: https://doi.org/10.1101/524793; this version posted January 19, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Direct binding of the flexible C-terminal segment of periaxin to β4 integrin suggests 2 a molecular basis for CMT4F 3 4 Arne Raasakka1, Helen Linxweiler1, Peter J. Brophy2, Diane L. Sherman2,*, Petri 5 Kursula1,3,* 6 7 1Department of Biomedicine, University of Bergen, Bergen, Norway 8 2Centre for Discovery Brain Sciences, University of Edinburgh, Edinburgh, United 9 Kingdom 10 3Faculty of Biochemistry and Molecular Medicine, University of Oulu, Oulu, Finland 11 12 *Corresponding authors: Diane Sherman, [email protected]; Petri Kursula, 13 [email protected] 14 15 16 1 bioRxiv preprint doi: https://doi.org/10.1101/524793; this version posted January 19, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Abstract 2 3 The process of myelination in the nervous system requires coordinated formation of 4 both transient and stable supramolecular complexes. Myelin-specific proteins play 5 key roles in these assemblies, which may link membranes to each other or connect 6 the myelinating cell cytoskeleton to the extracellular matrix. The myelin protein 7 periaxin is known to play an important role in linking the Schwann cell cytoskeleton 8 to the basal lamina through membrane receptors, such as the dystroglycan complex.