Properties of Gases a Chem1 Supplement Text Stephen K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gases and Pressure Changes
11.2SECTION Gases and Pressure Changes If you have fl own in an airplane, you may have felt discomfort in your ears during Key Terms take-off or landing. As soon as your ears “popped,” they probably felt better. A similar atmospheric pressure experience is common while riding up and down on an elevator. Your ears become blocked and then unblocked due to changes in atmospheric pressure. Although you are standard atmospheric usually unaware of the eff ect of atmospheric pressure on your body, the atmosphere is pressure (SAP) always exerting a large amount of pressure on you from all directions. Boyle’s law To describe and explain the behaviour of the gases in Earth’s atmosphere, as well as the behaviour of other gases, you need to understand the meanings of pressure and gas pressure. Pressure is the force that is exerted on an object per unit of surface area. Th e phrase “per unit of surface area” means the area over which the force is distributed. Th e equation for pressure is force F pressure = _ or P = _ area A Th e SI unit for force is the newton (N), and the unit for area is the square metre (m2). Th erefore, the corresponding unit of pressure is newtons per square metre (N/m2). Later in this chapter, you will learn how this unit for pressure is related to other commonly used units for presssure, such as the pascal (Pa) and millimetres of mercury (mmHg). Atmospheric Pressure Earth’s atmosphere is a spherical envelope of gases that surrounds the planet and atmospheric pressure extends from Earth’s surface outward to space. -

Pressure and Piezometry (Pressure Measurement)
PRESSURE AND PIEZOMETRY (PRESSURE MEASUREMENT) What is pressure? .......................................................................................................................................... 1 Pressure unit: the pascal ............................................................................................................................ 3 Pressure measurement: piezometry ........................................................................................................... 4 Vacuum ......................................................................................................................................................... 5 Vacuum generation ................................................................................................................................... 6 Hydrostatic pressure ...................................................................................................................................... 8 Atmospheric pressure in meteorology ...................................................................................................... 8 Liquid level measurement ......................................................................................................................... 9 Archimedes' principle. Buoyancy ............................................................................................................. 9 Weighting objects in air and water ..................................................................................................... 10 Siphons ................................................................................................................................................... -

CAR-ANS PART 05 Issue No. 2 Units of Measurement to Be Used In
CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES Part 5 Governing UNITS OF MEASUREMENT TO BE USED IN AIR AND GROUND OPERATIONS CIVIL AVIATION AUTHORITY OF THE PHILIPPINES Old MIA Road, Pasay City1301 Metro Manila INTENTIONALLY LEFT BLANK CAR-ANS PART 5 Republic of the Philippines CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES (CAR-ANS) Part 5 UNITS OF MEASUREMENTS TO BE USED IN AIR AND GROUND OPERATIONS 22 APRIL 2016 EFFECTIVITY Part 5 of the Civil Aviation Regulations-Air Navigation Services are issued under the authority of Republic Act 9497 and shall take effect upon approval of the Board of Directors of the CAAP. APPROVED BY: LT GEN WILLIAM K HOTCHKISS III AFP (RET) DATE Director General Civil Aviation Authority of the Philippines Issue 2 15-i 16 May 2016 CAR-ANS PART 5 FOREWORD This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was formulated and issued by the Civil Aviation Authority of the Philippines (CAAP), prescribing the standards and recommended practices for units of measurements to be used in air and ground operations within the territory of the Republic of the Philippines. This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was developed based on the Standards and Recommended Practices prescribed by the International Civil Aviation Organization (ICAO) as contained in Annex 5 which was first adopted by the council on 16 April 1948 pursuant to the provisions of Article 37 of the Convention of International Civil Aviation (Chicago 1944), and consequently became applicable on 1 January 1949. The provisions contained herein are issued by authority of the Director General of the Civil Aviation Authority of the Philippines and will be complied with by all concerned. -

The Mercury Sphygmomanometer Should Be Abandoned Before It Is Proscribed
Journal of Human Hypertension (2000) 14, 31–36 2000 Macmillan Publishers Ltd. All rights reserved 0950-9240/00 $15.00 www.nature.com/jhh ORIGINAL ARTICLE The mercury sphygmomanometer should be abandoned before it is proscribed ND Markandu, F Whitcher, A Arnold and C Carney Blood Pressure Unit, Department of Medicine, St George’s Hospital Medical School, London SW17 ORE, UK Both in clinical practice and medical research, blood cury is a non-degradable pollutant, eventually accumu- pressure is still largely measured by auscultation using lating on the sea bed. The use of mercury in sphygmo- a mercury sphygmomanometer. Blood pressure is the manometers is already in the process of being most important predictor of life expectancy. Treatment eliminated in Scandinavia and Holland and other coun- of high blood pressure reduces strokes, heart attack tries are likely to follow. Our results suggest that mer- and heart failure. Accurate measurement is therefore cury sphygmomanometers are not adequately main- essential. At a large London teaching hospital, just tained and require expertise that is not available for under 500 mercury sphygmomanometers and their accurate measurement of blood pressure. Their use associated cuffs were examined. More than half had should be dispensed with on these grounds before a serious problems that would have rendered them inac- ban for other and, perhaps less justifiable reasons. Vali- curate in measuring blood pressure. At the same time, dated automatic devices, which are less liable to assessment of the technical knowledge needed to mea- measurement and observer error should be used sure blood pressure by the ausculatory technique was instead. -
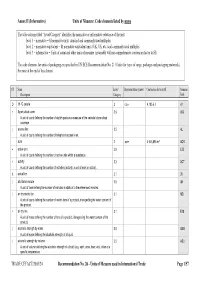
Units of Measure Used in International Trade Page 1/57 Annex II (Informative) Units of Measure: Code Elements Listed by Name
Annex II (Informative) Units of Measure: Code elements listed by name The table column titled “Level/Category” identifies the normative or informative relevance of the unit: level 1 – normative = SI normative units, standard and commonly used multiples level 2 – normative equivalent = SI normative equivalent units (UK, US, etc.) and commonly used multiples level 3 – informative = Units of count and other units of measure (invariably with no comprehensive conversion factor to SI) The code elements for units of packaging are specified in UN/ECE Recommendation No. 21 (Codes for types of cargo, packages and packaging materials). See note at the end of this Annex). ST Name Level/ Representation symbol Conversion factor to SI Common Description Category Code D 15 °C calorie 2 cal₁₅ 4,185 5 J A1 + 8-part cloud cover 3.9 A59 A unit of count defining the number of eighth-parts as a measure of the celestial dome cloud coverage. | access line 3.5 AL A unit of count defining the number of telephone access lines. acre 2 acre 4 046,856 m² ACR + active unit 3.9 E25 A unit of count defining the number of active units within a substance. + activity 3.2 ACT A unit of count defining the number of activities (activity: a unit of work or action). X actual ton 3.1 26 | additional minute 3.5 AH A unit of time defining the number of minutes in addition to the referenced minutes. | air dry metric ton 3.1 MD A unit of count defining the number of metric tons of a product, disregarding the water content of the product. -

Insect Epicuticle a Name Given to the Millimetre of Mercury Pressure As a Unit of Pressure
No. 3940, MAY 5, 1945 NATURE 545 control of eelworm may be obtained, and this in Units for Degree of Vacuum addition to the already established control of onion fly and onion white rot, it would seem that the use IT has long been considered in this laboratory that of calomel in respect of onion crops may be very when working in the field of medium and high vacua valuable. (pressures of 1 mm. mercury or less), the usual F. 0. MosLEY. methods of referring to and recording the degree of Research Laboratory, vacuum are extremely awkward verbally and little The Nurseries, less so when written. Uxbridge. There are a number of known systems of recording 1 such pressures, the bar and the millimetre of mercury Nature, 155, 241 (1945). pressure (mm. Hg) and their subdivisions being most widely known, the micron being a of mm. and the Torr, found in German practiCe, bemg Insect Epicuticle a name given to the millimetre of mercury pressure as a unit of pressure. The millimetre of mercury pressure Alexander, Kitchene · and Briscoe1• 2 have shown is almost universally used, but when subdivisions of that the desiccation of many insects caused by inert this unit are used, recourse is necessarily made to dust insecticides is due to adsorption of the epicuticle either the negative index method or the decimal wax film, which becomes discontinuous and allows of method of reference or recording. Thus, a pressure increased loss of water through the cuticle. Further, of, say, 2 x J0-5 mm. mercury may be written so, Wigglesworth"·' has demonstrated abrasion of the or as 0 ·00002 mm. -

146 NATURE August 4, 1945 Vol
146 NATURE August 4, 1945 Vol. 156 In conclusion, we should like to thank Dr. T. converted to millibars, etc., and this labour might Swarbrick (Long Ashton Research Station), Mr. G. M. present the chief obstacle to the adoption of the Stuart (East of Scotland Agricultural College), and proposal. (c) A very large number of constants Messrs. Chandler and Dunn (Canterbury), who kindly including the International Temperature Scale and provided some of the specimens. tables of boiling-points, the values of which depend A. BERYL BEAKBANE. on pressure and are given for a specified pressure. ELEANOR C. THOMPSON. In most cases there is no need to recalculate these East Malling Research Station, quantities immediately-they can be quoted for Kent. May 30. l ·01323 bar instead of for "760 mm. Hg at 0° C. and lat. 45°." 1 Wallace, T., Swarbr!ck, T., and Ogilvie, L., Grower, 22 (49), 12 (1944). Finally, for approximate conversion from units of ' Crane, M. B., Grower, 22, (53), 10 (1944). mercury pressure to absolute units, it may be noted • Crane, M. B., Nature, 155, 115 (1945). that 1 bar is equivalent to 750·08 mm. of mercury 'Rendle, B. J., Tropical Woodt, 152, 11 (1937), (approx. 75 cm.-a point which may interest those who teach elementary science). E. DENNE. Pressure Units Science Department, Mid-Essex Technical College, THE recent communication by Dr. W. R. G. Chelmsford. Atkins1 directs attention to a considerable amount of confusion which still exists in statements of num 1 Atkins, W. R. G., Nat1tre, 155, 361 (1945). erical results and constants. -

Siunitx – a Comprehensive (Si) Units Package∗
siunitx – A comprehensive (si) units package∗ Joseph Wright† Released 2021-09-22 Contents 1 Introduction 3 2 siunitx for the impatient 3 3 Using the siunitx package 4 3.1 Numbers . 4 3.2 Angles . 5 3.3 Units . 6 3.4 Complex numbers and quantities . 7 3.5 The unit macros . 8 3.6 Unit abbreviations . 10 3.7 Creating new macros . 14 3.8 Tabular material . 15 4 Package control options 17 4.1 The key–value control system . 17 4.2 Printing . 18 4.3 Parsing numbers . 20 4.4 Post-processing numbers . 22 4.5 Printing numbers . 25 4.6 Lists, products and ranges . 28 4.7 Complex numbers . 31 4.8 Angles . 32 4.9 Creating units . 34 4.10 Using units . 35 4.11 Quantities . 38 4.12 Tabular material . 39 4.13 Locale options . 49 4.14 Preamble-only options . 49 5 Upgrading from version 2 49 6 Unit changes made by BIPM 51 ∗This file describes v3.0.31, last revised 2021-09-22. †E-mail: [email protected] 1 7 Localisation 52 8 Compatibility with other packages 52 9 Hints for using siunitx 52 9.1 Adjusting \litre and \liter ........................ 52 9.2 Ensuring text or math output . 53 9.3 Expanding content in tables . 53 9.4 Using siunitx with datatool .......................... 54 9.5 Using units in section headings and bookmarks . 55 9.6 A left-aligned column visually centred under a heading . 56 9.7 Regression tables . 57 9.8 Maximising performance . 58 9.9 Special considerations for the \kWh unit . -
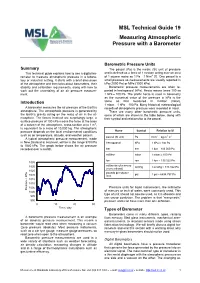
TG19 JUNE 2009 Version
MSL Technical Guide 19 Measuring Atmospheric Pressure with a Barometer Barometric Pressure Units Summary The pascal (Pa) is the metric (SI) unit of pressure This technical guide explains how to use a digital ba- and is defined as a force of 1 newton acting over an area 2 rometer to measure atmospheric pressure in a labora- of 1 square metre so 1 Pa = 1 N/m [1]. One pascal is a tory or industrial setting. It starts with a brief discussion small pressure so measurements are usually reported in of the atmosphere and then talks about barometers, their kPa (1000 Pa) or MPa (1000 kPa). stability and calibration requirements, along with how to Barometric pressure measurements are often re- work out the uncertainty of an air pressure measure- ported in hectopascal (hPa). Hecto means times 100 so ment. 1 hPa = 100 Pa. The prefix hecto is used in barometry as the numerical value of the pressure in hPa is the Introduction same as that measured in millibar (mbar), 1 mbar = 1 hPa = 100 Pa. Many historical meteorological A barometer measures the air pressure of the Earth’s records of atmospheric pressure were recorded in mbar. atmosphere. The atmospheric pressure is generated by There are many other barometric pressure units, the Earth’s gravity acting on the mass of air in the at- some of which are shown in the table below, along with mosphere. The forces involved are surprisingly large, a their symbol and relationship to the pascal. surface pressure of 100 kPa means the force at the base 2 of a column of the atmosphere, cross-section area 1 m , is equivalent to a mass of 10,000 kg. -

Vacuum Processing of Food–A Mini Review
MOJ Food Processing & Technology Mini Review Open Access Vacuum processing of food–a Mini Review Abstract Volume 6 Issue 3 - 2018 Conventional food processing operations such as frying, drying, and blanching which Sharanabasava, Menon Rekha Ravindra are performed under atmospheric condition sexposures product to high processing SRS of ICAR, India temperature. In addition to the potential product contamination from exposure to atmospheric conditions, other challenges/issues include nutrient losses, changes in Correspondence: Menon Rekha Ravindra, SRS of ICAR– the product sensory attributes and lengthy processing time. In order to minimize the NDRI, Bangalore, India, Email [email protected] aforementioned disadvantages, the food industry has adopted novel techniques like vacuum processing for operations such as frying, cooling, drying, packaging etc. Received: December 02, 2016 | Published: May 25, 2018 Keywords: food processing, vacuum pressure, standard atmosphere, lacking, food products, vegetables, kiwifruits Introduction a vacuum frying system. The fried product was centrifuged at 450 rpm within the frying chamber. Sothornvit3 studied different patterns The term vacuum is generally used to represent a volume or state of vacuum frying system used in processing. He developed a vacuum of space in which the pressure is considerably less than atmospheric frying chamber equipped with heated oil from another chamber, pressure. In the pressure measurement system, normal vacuum a cooling tower type condenser and an oil‒sealed vacuum pump. pressure is expressed in millimeters of a column of mercury, and Diamante et al.4 used a vacuum frying chamber with a water‒cooled 760millimeters of mercury is designated as 1 standard atmosphere. condenser cooling tower and liquid ring vacuum pump to vacuum Another conventional unit of pressure, especially at lower pressure fry jackfruit chips. -
O'brien E. Consequences of Banning Mercury and the Cuff Controversy
PROCEEDINGS OF A WORKSHOP ON BPM Consequences of banning mercury and the cuff controversy Eoin O’Brien Blood Pressure Monitoring 2000, 5:33-34 Banning mercury Blood Pressure Unit, Beaumont Hospital, Dublin, Ireland Mercury will be banned from clinical use in the near future because it is a toxic, persistent and bio-accuma- Correspondence to Professor Eoin O’Brien, The Blood Pressure Unit, ble substance, many tons of which are distributed Beaumont Hospital, Dublin 9, Ireland. Tel: f353 1 809 2160; fax: +353 1 809 3005; throughout the world to hospitals and countless individ- e-mail: [email protected] ual doctors and little of which is returned for disposal. It finds its way back into the environment through evap- b oration, in sewage or in solid waste, most seriously damaging the marine environment, and it accumulates in soil and in sediments thereby entering the food chain [l]. Signatories of the ‘Final Declaration from the Third International Conference on the Protection of the North Sea’ have resolved to reduce environmental mercury to ‘levels that are not harmful to man or nature before the year 2000’ [l]. One of the consequences of the impending ban on mercury is that authoritative bodies, government health agencies and purchasing authorities have been reluctant to make recommendations and give guidance to doctors and the public. Rather it falls upon bodies such as The Working Group on Blood Pressure Monitoring to evalu- ate practice and make recommendations. The result, however, of this ambivalence is that hospitals and doc- tors may replace mercury sphygmomanometers by unre- liable and inaccurate devices, such as aneroid sphygmo- manometers, which become inaccurate with use and should not, therefore, be substituted for the mercury instrument [Z]. -
Beyond the Metre (Part III)
Iowa Science Teachers Journal Volume 18 Number 1 Article 7 1981 Beyond the Metre (Part III) Richard S. Thompkins Mississippi Bend Area Education Agency Follow this and additional works at: https://scholarworks.uni.edu/istj Part of the Science and Mathematics Education Commons Let us know how access to this document benefits ouy Copyright © Copyright 1981 by the Iowa Academy of Science Recommended Citation Thompkins, Richard S. (1981) "Beyond the Metre (Part III)," Iowa Science Teachers Journal: Vol. 18 : No. 1 , Article 7. Available at: https://scholarworks.uni.edu/istj/vol18/iss1/7 This Article is brought to you for free and open access by the Iowa Academy of Science at UNI ScholarWorks. It has been accepted for inclusion in Iowa Science Teachers Journal by an authorized editor of UNI ScholarWorks. For more information, please contact [email protected]. BEYOND THE METRE (Part III) Richard S. Tompkins Metric Education Consultant Mississippi Bend Area Education Agency 2604 West Locust Street Davenport, Iowa 52804 Introduction This article is the third in a series discussing the wide spectrum of metric units. The first dealt with some general aspects of the Interna tional System of Units (SI) and then went on to consider the metre, square metre, cubic metre and second. The second article described the kilogram and the metric units of velocity and acceleration. This article deals with metric units of density, force and pressure. The Kilogram Per Cubic Metre (kg/m3) The concept of density originated in the Middle Ages. (1) By itself, the term refers to the mass contained in a single volume unit of the material under consideration.