Marine Fish Osteology a Manual for Archaeologists
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Possibilities of Osteology in Historical Sarni Archaeology Th Life and Livelihood at the 18 -Century Ohcejohka Sarni Market Site
The possibilities of osteology in historical Sarni archaeology th Life and livelihood at the 18 -century Ohcejohka Sarni market site Eeva-Kristiina Harlin Giellagas Institute, Porotie 12, Fl- 99950 Karigasniemi, Finland Abstract The Ohcejohka market site This paper presents the archaeological material The Ohcejohka market site is well known from from a historical Sarni market site in Ohcejohka. written sources. In the past, it was the central The site was in use already in 1640 when annual place for the Ohcejohka siida (Lapp village), markets were held in the area, and the Ohcejoh- and annual markets were held there at the end ka church was erected at the site in 1701. The of February already in 1640. Due to the colonial excavated material derives from two traditional policy of the Swedish crown, the Ohcejohka and Sarni huts, goahti. The find material is quite typi- Guovdageaidnu churches were erected in 1701, cal for l ?1"- l 9th-century Sarni sites, and the main and even today the new Ohcejohka church, find group consists of unburned animal bones. erected between 1850 and 1853, is situated near The animal bones are analysed and questions of the site (ltkonen 1948 I: 206- 208, 303; 1948 II: livelihood are discussed. 59, 203). Additionally, there is an old sacristy and cemetery at the site and a historical road Keywords: to the Norwegian coast passes through the area Sarni studies, osteology, ethnoarchaeology, his- (Karjalainen 2003). torical archaeology, reindeer. During the winter markets, both live reindeer and reindeer products were sold by the Sarni and traded with burghers coming from southern Introduction towns. -

BONY FISHES 602 Bony Fishes
click for previous page BONY FISHES 602 Bony Fishes GENERAL REMARKS by K.E. Carpenter, Old Dominion University, Virginia, USA ony fishes constitute the bulk, by far, of both the diversity and total landings of marine organisms encoun- Btered in fisheries of the Western Central Atlantic.They are found in all macrofaunal marine and estuarine habitats and exhibit a lavish array of adaptations to these environments. This extreme diversity of form and taxa presents an exceptional challenge for identification. There are 30 orders and 269 families of bony fishes presented in this guide, representing all families known from the area. Each order and family presents a unique suite of taxonomic problems and relevant characters. The purpose of this preliminary section on technical terms and guide to orders and families is to serve as an introduction and initial identification guide to this taxonomic diversity. It should also serve as a general reference for those features most commonly used in identification of bony fishes throughout the remaining volumes. However, I cannot begin to introduce the many facets of fish biology relevant to understanding the diversity of fishes in a few pages. For this, the reader is directed to one of the several general texts on fish biology such as the ones by Bond (1996), Moyle and Cech (1996), and Helfman et al.(1997) listed below. A general introduction to the fisheries of bony fishes in this region is given in the introduction to these volumes. Taxonomic details relevant to a specific family are explained under each of the appropriate family sections. The classification of bony fishes continues to transform as our knowledge of their evolutionary relationships improves. -

Bioarchaeology (Anthropological Archaeology) - Mario ŠLAUS
PHYSICAL (BIOLOGICAL) ANTHROPOLOGY - Bioarchaeology (Anthropological Archaeology) - Mario ŠLAUS BIOARCHAEOLOGY (ANTHROPOLOGICAL ARCHAEOLOGY) Mario ŠLAUS Department of Archaeology, Croatian Academy of Sciences and Arts, Zagreb, Croatia. Keywords: Bioarchaeology, archaeological, forensic, antemortem, post-mortem, perimortem, traumas, Cribra orbitalia, Harris lines, Tuberculosis, Leprosy, Treponematosis, Trauma analysis, Accidental trauma, Intentional trauma, Osteological, Degenerative disease, Habitual activities, Osteoarthritis, Schmorl’s nodes, Tooth wear Contents 1. Introduction 1.1. Definition of Bioarchaeology 1.2. History of Bioarchaeology 2. Analysis of Skeletal Remains 2.1. Excavation and Recovery 2.2. Human / Non-Human Remains 2.3. Archaeological / Forensic Remains 2.4. Differentiating between Antemortem/Postmortem/Perimortem Traumas 2.5. Determination of Sex 2.6. Determination of Age at Death 2.6.1. Age Determination in Subadults 2.6.2. Age Determination in Adults. 3. Skeletal and dental markers of stress 3.1. Linear Enamel Hypoplasia 3.2. Cribra Orbitalia 3.3. Harris Lines 4. Analyses of dental remains 4.1. Caries 4.2. Alveolar Bone Disease and Antemortem Tooth Loss 5. Infectious disease 5.1. Non–specific Infectious Diseases 5.2. Specific Infectious Disease 5.2.1. Tuberculosis 5.2.2. Leprosy 5.2.3. TreponematosisUNESCO – EOLSS 6. Trauma analysis 6.1. Accidental SAMPLETrauma CHAPTERS 6.2. Intentional Trauma 7. Osteological and dental evidence of degenerative disease and habitual activities 7.1. Osteoarthritis 7.2. Schmorl’s Nodes 7.3. Tooth Wear Caused by Habitual Activities 8. Conclusion Glossary Bibliography Biographical Sketch ©Encyclopedia of Life Support Systems (EOLSS) PHYSICAL (BIOLOGICAL) ANTHROPOLOGY - Bioarchaeology (Anthropological Archaeology) - Mario ŠLAUS 1. Introduction 1.1. Definition of Bioarchaeology Bioarchaeology is the study of human biological remains within their cultural (archaeological) context. -

Preliminary Mass-Balance Food Web Model of the Eastern Chukchi Sea
NOAA Technical Memorandum NMFS-AFSC-262 Preliminary Mass-balance Food Web Model of the Eastern Chukchi Sea by G. A. Whitehouse U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service Alaska Fisheries Science Center December 2013 NOAA Technical Memorandum NMFS The National Marine Fisheries Service's Alaska Fisheries Science Center uses the NOAA Technical Memorandum series to issue informal scientific and technical publications when complete formal review and editorial processing are not appropriate or feasible. Documents within this series reflect sound professional work and may be referenced in the formal scientific and technical literature. The NMFS-AFSC Technical Memorandum series of the Alaska Fisheries Science Center continues the NMFS-F/NWC series established in 1970 by the Northwest Fisheries Center. The NMFS-NWFSC series is currently used by the Northwest Fisheries Science Center. This document should be cited as follows: Whitehouse, G. A. 2013. A preliminary mass-balance food web model of the eastern Chukchi Sea. U.S. Dep. Commer., NOAA Tech. Memo. NMFS-AFSC-262, 162 p. Reference in this document to trade names does not imply endorsement by the National Marine Fisheries Service, NOAA. NOAA Technical Memorandum NMFS-AFSC-262 Preliminary Mass-balance Food Web Model of the Eastern Chukchi Sea by G. A. Whitehouse1,2 1Alaska Fisheries Science Center 7600 Sand Point Way N.E. Seattle WA 98115 2Joint Institute for the Study of the Atmosphere and Ocean University of Washington Box 354925 Seattle WA 98195 www.afsc.noaa.gov U.S. DEPARTMENT OF COMMERCE Penny. S. Pritzker, Secretary National Oceanic and Atmospheric Administration Kathryn D. -

Blackchin Tilapia (Sarotherodon Melanotheron) Ecological Risk Screening Summary
U.S. Fish and Wildlife Service Blackchin Tilapia (Sarotherodon melanotheron) Ecological Risk Screening Summary Web Version – 10/01/2012 Photo: © U.S. Geological Survey From Nico and Neilson (2014). 1 Native Range and Nonindigenous Occurrences Native Range From Nico and Neilson (2014): “Tropical Africa. Brackish estuaries and lagoons from Senegal to Zaire (Trewavas 1983).” Nonindigenous Occurrences From Nico and Neilson (2014): “Established in Florida and Hawaii. Evidence indicates it is spreading rapidly in both fresh and salt water around island of Oahu, Hawaii (Devick 1991b).” “The first documented occurrence of this species in Florida was a specimen gillnetted by commercial fishermen in Hillsborough Bay near Tampa, Hillsborough County, in 1959 (Springer and Finucane 1963). Additional records for the western part of the state indicate that this species is established in brackish and freshwaters in eastern Tampa Bay and in adjoining drainages in Hillsborough County, ranging from the Alafia River south to Cockroach Bay. The species has been recorded from the Alafia River from its mouth up to Lithia Springs; from the Hillsborough River, Bullfrog Creek, the Palm River, and the Little Manatee River; and from various western drainage and irrigation ditches (Springer and Finucane 1963; Finucane and Rinckey 1967; Buntz Sarotherodon melanotheron Ecological Risk Screening Summary U.S. Fish and Wildlife Service – Web Version – 10/01/2012 and Manooch 1969; Lachner et al. 1970; Courtenay et al. 1974; Courtenay and Hensley 1979; Courtenay and Kohler 1986; Lee et al. 1980 et seq.; Courtenay and Stauffer 1990; DNR collections; UF museum specimens). There are two records of this species from the west side of Tampa Bay, in Pinellas County: a collection from Lake Maggiore in St. -

Carpals and Tarsals of Mule Deer, Black Bear and Human: an Osteology Guide for the Archaeologist
Western Washington University Western CEDAR WWU Graduate School Collection WWU Graduate and Undergraduate Scholarship 2009 Carpals and tarsals of mule deer, black bear and human: an osteology guide for the archaeologist Tamela S. Smart Western Washington University Follow this and additional works at: https://cedar.wwu.edu/wwuet Part of the Anthropology Commons Recommended Citation Smart, Tamela S., "Carpals and tarsals of mule deer, black bear and human: an osteology guide for the archaeologist" (2009). WWU Graduate School Collection. 19. https://cedar.wwu.edu/wwuet/19 This Masters Thesis is brought to you for free and open access by the WWU Graduate and Undergraduate Scholarship at Western CEDAR. It has been accepted for inclusion in WWU Graduate School Collection by an authorized administrator of Western CEDAR. For more information, please contact [email protected]. MASTER'S THESIS In presenting this thesis in partial fulfillment of the requirements for a master's degree at Western Washington University, I grant to Western Washington University the non-exclusive royalty-free right to archive, reproduce, distribute, and display the thesis in any and all forms, including electronic format, via any digital library mechanisms maintained by WWu. I represent and warrant this is my original work, and does not infringe or violate any rights of others. I warrant that I have obtained written permissions from the owner of any third party copyrighted material included in these files. I acknowledge that I retain ownership rights to the copyright of this work, including but not limited to the right to use all or part of this work in future works, such as articles or books. -

Pontoniine Shrimps
Pontoniine shrimps (Decapoda: Caridea: Palaemonidae) inhabiting boring sponges (Porifera: Demospongia) from Nhatrang Bay, Vietnam, with descriptions of three new species I. Marin Marin, I. Pontoniine shrimps (Decapoda: Caridea: Palaemonidae) inhabiting boring sponges (Porifera: Demospongia) from Nhatrang Bay, Vietnam, with description of three new species. Zool. Med. Leiden 81 (12), 8.vi.2007: 217-240, fi gs 1-18.— ISSN 0024-0672. Ivan Marin, Laboratory of Ecology and Morphology of Marine Invertebrates, A.N. Severtzov Institute of Ecology and Evolution RAS, Leninsky prospect, 33, Moscow, 117071, Russia. Coastal Department of Russian-Vietnamese Tropical Center, Nguyen Thien Thuat, 30, Nha Trang City, Vietnam (e-mail: coral- [email protected]). Key words: Crustacea; Decapoda; Palaemonidae; Pontoniinae; Apopontonia, Onycocaridella, Onycocaris, Periclimenaeus, Poripontonia; new species, new records; sponges, symbiosis; Vietnam. Some further investigations on symbiotic fauna of shallow-water boring demosponges in Nhatrang Bay, Vietnam are given. Three species of pontoniine shrimps are described as new: Onycocaridella an- tokha spec. nov., Periclimenaeus pachyspinosus spec. nov., and Poripontonia cornuta spec. nov. Four spe- cies, Apopontonia falcirostris Bruce, 1976, Onycocaris amasukensis Fujino & Miyake, 1969, Periclimenaeus djiboutensis Bruce, 1970 and Periclimenaeus rastrifer Bruce, 1980, are recorded from Vietnam for the fi rst time. Introduction The symbiotic fauna associated with shallow-water demosponges is very rich. It is based on the presence of a developed canal system with a continuous fl ow of water (Bergquist, 1978) and the perfect shelter of the inhabitants by toxicity of the sponges for large predators (Bakhus, 1981). Most of the epibiotic species are hydroids (Puce et al, 2005) and polychaetes (Martin & Britayev, 1998) while endobiotic alpheid (Decapoda: Caridea: Alpheidae) and palaemonid (Decapoda: Palaemonidae) shrimps are the most diverse groups inside sponges. -

Subdisciplines of Anthropology: a Modular Approach
DOCUMENT RESUME ED 260 774 JC 850 487 AUTHOR Kassebaum, Peter TITLE Subdisciplines of Anthropology: A Modular Approach. Cultural Anthropology. INSTITUTION College of Marin, Kentfield, Calif. PUB DATE ) [84] NOTE 19p. PUB TYPE Guides - Classroom Use- Materials (For Learner) (051) EDRS PRICE ,MF01/PC01 Plus Postage. DESCRIPTORS *Anthropology; Community Colleges; *Intellectual Disciplines; Learning Modules; TwoYear Colleges IDENTIFIERS *Cultural Anthropology ABSTRACT Designed for mse as supplementary instructional material in 4 cultural anthropologycourse; this learning module introduces the idea that anthropology iscomposed of a number of subdisciplines and that cultural amthropologyhas numerous subfields which are thg specialtyareas for many practicing anthropologists. Beginning with a general discussion ofthe field of anthropology, the paper next describes, defines, and discusses theoreticaland historical considerations, for the followingsubdisciplines within anthropology:. (1) archaeology; (2) physicalanthropology; (3) medical anthropology; (4) cultural anthropology; (5)ethnology; (6) =mathematical anthropology; (7),economicanthropology; (8) political anthropology; (9) the ethnography of law; (10)anthropology and education; (11) linguistics; (12) folklore; (13)ethnomusicology; (14) art and anthropology; (15) *nthropologyand belief systems; (16) culture and perionality; (17)-appliedanthropology; (18) urban anthropology; and,(1,9) economic anthropology.A test for students is included. (LAL) %N. ***************************************w******************************* -

Southampton French Quarter 1382 Specialist Report Download E2: Fish Bone
Southampton French Quarter SOU1382 Specialist Report Download E2 Southampton French Quarter 1382 Specialist Report Download E2: Fish Bone By Rebecca Nicholson Introduction An assemblage of almost 7500 identifiable fish bones was recovered both by hand retrieval during the excavation, but predominantly from the sorted residues of the processed bulk soil samples. During excavations at Southampton French Quarter a total of 188 bulk samples were sieved to 0.5mm (occasionally 1mm) as part of the flotation process for the recovery of plant and animal remains. The sampling strategy followed during the excavation involved, where possible, the full sampling of one rubbish pit and one latrine pit per tenement for each major period, avoiding intercut features or those clearly containing residual material. Occupation surfaces and other distinct features such as hearths were also sampled. Mixed contexts or contexts of uncertain provenance were avoided. While complete standardisation of sample volumes was not possible, wherever practicable samples were 40 litres. Following assessment of the fish remains recovered largely from the > 4mm residues, the richer assemblages were targeted for further fine residue and flot sorting. This report comprises an analysis of all the identified the fish remains from these samples, together with the material collected by hand on site. Methodology The residues from all of the bulk-sieved samples were sorted to 4mm. Where samples were identified as having significant numbers of fish bones, residues were sorted to 2mm. Samples from the Late Saxon deposits which produced fish remains were routinely sorted to 2mm even where fish remains were not abundant, in order to avoid a perceived bias against the recovery of small fish in pre-medieval deposits (see Barrett et al. -

INVERTEBRATE SPECIES in the EASTERN BERING SEA By
Effects of areas closed to bottom trawling on fish and invertebrate species in the eastern Bering Sea Item Type Thesis Authors Frazier, Christine Ann Download date 01/10/2021 18:30:05 Link to Item http://hdl.handle.net/11122/5018 e f f e c t s o f a r e a s c l o s e d t o b o t t o m t r a w l in g o n fish a n d INVERTEBRATE SPECIES IN THE EASTERN BERING SEA By Christine Ann Frazier RECOMMENDED: — . /Vj Advisory Committee Chair Program Head / \ \ APPROVED: M--- —— [)\ Dean, School of Fisheries and Ocean Sciences • ~7/ . <-/ / f a Dean of the Graduate Sch6oI EFFECTS OF AREAS CLOSED TO BOTTOM TRAWLING ON FISH AND INVERTEBRATE SPECIES IN THE EASTERN BERING SEA A THESIS Presented to the Faculty of the University of Alaska Fairbanks in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE 6 By Christine Ann Frazier, B.A. Fairbanks, Alaska December 2003 UNIVERSITY OF ALASKA FAIRBANKS ABSTRACT The Bering Sea is a productive ecosystem with some of the most important fisheries in the United States. Constant commercial fishing for groundfish has occurred since the 1960s. The implementation of areas closed to bottom trawling to protect critical habitat for fish or crabs resulted in successful management of these fisheries. The efficacy of these closures on non-target species is unknown. This study determined if differences in abundance, biomass, diversity and evenness of dominant fish and invertebrate species occur among areas open and closed to bottom trawling in the eastern Bering Sea between 1996 and 2000. -
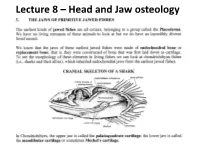
Lecture 8 – Head and Jaw Osteology
Lecture 8 – Head and Jaw osteology More derived fishes (Ray finned fishes) The variability of the jaw structure of bony fishes provides an explanation for the extensive adaptive radiation in the group and why they are so diverse and occupy almost every aquatic niche available. Skull diversity (A) carp, Cyprinus carpio, (B) vampire characin, Hydrolycus scomberoides, (C) catfish Arius felis. (D) cod Gadus morhua. (E) large-mouth bass, Micropterus salmoides (F) The parrotfish Scarus guacamaia. Scale bar = 10 mm WESTNEAT 2004 From an evolutionary standpoint, fishes were the first animals to develop bony jaws. Versatile jaws and multiple feeding strategies allowed fishes to fill, or radiate into, a diverse range of niches. They have evolved to feed in all possible ways – sucking, biting, scraping, nipping, crushing etc. The head of a teleost has 5 main regions: Cranium, jaws, cheeks, hydroid arch, opercula. The head of a fish has five main regions • 1) The CRANIUM is composed of the bones providing direct support and protection to the brain and the visual, Anterior Posterior olfactory, and auditory organs. Below the cranium is the parashenoid bone. • Parasphenoid plays a role in the jaws as Features of the neurocranium sensu lato (from Caranx it acts as a hard melampygus, lateral aspect, left, and posterior aspect, right). A = prevomer, B = ethmoid, C = frontal, D = palate supraoccipital, E = pterotic, F = exoccipital, G = basioccipital, H = foramen magnum, I = parasphenoid, J = orbit. The five main regions Bowfin 2) The JAWS • Lower Jaw – has an Angular articular and dentary bone • Angular articular- The paired bones form the posterior part of either side of the lower jaw and articulate with the suspensorium. -

Human Osteology ANT 3331-01/02/03 Spring, 2008 ANT 3331-01, Tuesday/Thursday, 9:30-10:50, 224 Marrs Mclean Science Bldg
Human Osteology ANT 3331-01/02/03 Spring, 2008 ANT 3331-01, Tuesday/Thursday, 9:30-10:50, 224 Marrs McLean Science Bldg. ANT 3331-02, Tuesday/Thursday, 11:00-12:20, 224 Marrs McLean Science Bldg. ANT 3331-03, Tuesday/Thursday, 12:30-1:50, 224 Marrs McLean Science Bldg. Instructor: Dr. Joseph Ferraro Office: 308.2 Marrs McLean Science Bldg. Phone: 710-1401 Email: [email protected] Office hours: Tuesday 3:00-5:00 in my office and/or in the lab, or by appointment. You can also reach me via phone and email. Remember, I’m here to help you learn: take advantage of me as a resource (within reason). Open lab hours: to be announced in class and posted on ‘Blackboard’ Texts: Required: Human Osteology. 2nd Ed. Tim D. White. Academic Press: New York. Strongly suggested: The Elements of Style. 4th Ed. William Strunk and E.B. White. Longman: Massachusetts (available almost everywhere, including the Baylor Bookstore). Course Overview: This class is designed to introduce you to the structure, design, and variability of the modern human skeleton. Much as the bony skeleton offers a framework for the rest of the body, so too will this course will provide a foundation for future studies in areas such as forensic sciences, physical anthropology, archaeology, and most aspects of medicine. For each element of the skeleton we will examine issues of structure, function, development, and evolutionary history. Lectures will also emphasize aspects of bone histology and biology, excavation and preservation, taphonomy, pathology, and the estimation of age and stature.