S. Coppens Et Al PROSPECT Hernia Repair 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Personalized Medicine for Reconstruction of Critical-Size Bone
www.nature.com/npjregenmed ARTICLE OPEN Personalized medicine for reconstruction of critical-size bone defects – a translational approach with customizable vascularized bone tissue ✉ Annika Kengelbach-Weigand 1 , Carolina Thielen 1, Tobias Bäuerle2, Rebekka Götzl 1,5, Thomas Gerber3, Carolin Körner4, Justus P. Beier1,5, Raymund E. Horch 1 and Anja M. Boos1,5 Tissue engineering principles allow the generation of functional tissues for biomedical applications. Reconstruction of large-scale bone defects with tissue-engineered bone has still not entered the clinical routine. In the present study, a bone substitute in combination with mesenchymal stem cells (MSC) and endothelial progenitor cells (EPC) with or without growth factors BMP-2 and VEGF-A was prevascularized by an arteriovenous (AV) loop and transplanted into a critical-size tibia defect in the sheep model. With 3D imaging and immunohistochemistry, we could show that this approach is a feasible and simple alternative to the current clinical therapeutic option. This study serves as proof of concept for using large-scale transplantable, vascularized, and customizable bone, generated in a living organism for the reconstruction of load-bearing bone defects, individually tailored to the patient’s needs. With this approach in personalized medicine for the reconstruction of critical-size bone defects, regeneration of parts of the human body will become possible in the near future. npj Regenerative Medicine (2021) 6:49 ; https://doi.org/10.1038/s41536-021-00158-8 1234567890():,; INTRODUCTION vascular networks consisting of endothelial cells can be created Therapeutic options for bone defects that cannot heal sponta- directly within tissue replacement materials7. Vascularization may neously, the so-called critical-size bone defects, are still limited be further supported by the addition of endothelial cells and and often associated with a great social burden. -
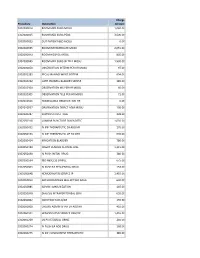
Procedure Description Charge Amount 0302000014 ROOM MED
Charge Procedure Description Amount 0302000014 ROOM MED SURG MOSU 1,040.00 0302000015 ROOM MED SURG PEDS 3,500.00 0302000033 OUT PATIENT BED MOSU 0.00 0302000035 ROOM INTERMEDIATE MOSU 2,055.00 0302000043 ROOM HOSPICE MOSU 805.00 0302000045 ROOM MED SURG W TELE MOSU 1,530.00 0302000050 OBSERVATION INTERM PER HR MOSU 97.00 0302002283 FECAL MANAGEMENT SYSTEM 694.00 0302010232 CATH INDWELL BLADDER SIMPLE 185.00 0302010304 OBSERVATION MS PER HR MOSU 60.00 0302010305 OBSERVATION TELE PER HR MOSU 71.00 0302010556 NONBILLABLE OBSERVATION HR 0.00 0302010557 OBSERVATION DIRECT ADM MOSU 130.00 0302020287 SUPPLIES CHEST TUBE 228.00 0302050318 LUMBAR PUNCTURE DIAGNOSTIC 1,035.00 0302050451 IV INF THERAPEUTIC EA ADD HR 170.00 0302050453 IV INF THERAPEUTIC UP TO 1HR 635.00 0302050454 IRRIGATION BLADDER 780.00 0302050480 INSERT VENOUS CENTRAL LINE 1,315.00 0302050490 IV PUSH INITIAL DRUG 380.00 0302050534 I&D ABSCESS SIMPLE 675.00 0302050603 IV PUSH EA SEQUENTIAL DRUG 153.00 0302050648 HEMODIALYSIS SERVICE IP 1,455.00 0302050813 ARTHROCENTESIS MAJ JNT WO IMAG 630.00 0302050885 ADMIN IMMUNIZATION 145.00 0302050948 DIALYSIS INTRAPERITONEAL SERV 655.00 0302060002 INJECTION SUB-Q/IM 155.00 0302060008 CHEMO ADMIN IV INF EA ADD HR 410.00 0302060101 HEMODIALYSIS SERVICE OBS/OP 1,455.00 0302060269 US PV RESIDUAL URINE 240.00 0302060274 IV PUSH EA ADD DRUG 168.00 0302060275 IV INF CONCURRENT THERAPEUTIC 385.00 0302060276 IV INF SEQUENTIAL THER UP TO 1 191.00 0302060293 INSERT STRAIGHT CATH THERAPEUT 185.00 0302060372 CHEMO ADMIN IV INF SEQ 1 HR 525.00 0302060373 -

116561 18 G IV Cath Needle, Sterile $5.00 116577 20 G IV Cath Needle
Charge Code Description Fee 116561 18 G IV Cath needle, sterile $5.00 116577 20 G IV Cath needle, sterile $5.00 116588 22 G IV Cath needle, sterile $5.00 118216 5% dextrose/NS (500 ml = 1 unit) $75.00 118237 5% Dextrose/Water, 250 ml $10.00 118229 5% Dextrose/Water, 500 ml $10.00 117453 Acet/Tylenol #3 with Codeine, 300mg, Oral $7.88 117515 Acet/Tylenol drops, 80mg $7.88 117525 Acet/Tylenol w/ Codeine, 12.5mg, Elixer $7.88 117467 Acet/Tylenol, 120mg, Suppository $7.88 117493 Acet/Tylenol, 325 mg, PO $7.88 117523 Acetaminophen/Tylenol Elixir, 160mg $7.88 117483 Acetaminophen/Tylenol, 160 mg, chewable tablet $7.88 117498 Acetaminophen/Tylenol, 500 mg tablet $7.88 117514 Acetaminophen/Tylenol, 60mg suppository $7.88 117528 Activated Charcoal, 25mg with Sorbital, Suspension $25.00 116826 Adaptic / Gel sheet / Kling for dermal or epidermal application, each $23.63 117046 Adenosine for therapeutic use, 6 mg (vial), IV $490.35 116958 Administration set, with small volume nonfiltered pneumatic nebulizer, disposable $35.00 117061 Adrenalin, Epinephrine, 0.1 mg, IV/IM/SC $42.00 117715 Adult-Fleet Enema $10.00 116967 Aerosol mask, used with DME nebulizer $15.00 117535 Afrin Nasal Spray (Oxymetazoline) $7.88 115336 Albumin; serum, plasma or whole blood $71.00 118279 Albuterol, inhalation solution, unit dose (per vial) $20.00 118293 Albuterol, up to 2.5 mg and ipratropium bromide, up to 0.5 mg, $40.00 115301 Alcohol Level, Blood (ETOH) $36.00 116615 Alcohol or peroxide, per pint $2.00 116076 Alligator Forceps $137.81 117538 Amidate / Etomidate 2mg, -

Annual Report
ANNUAL REPORT 2019 MARCH 2020 To Our Shareholders Alex Gorsky Chairman and Chief Executive Officer By just about every measure, Johnson & These are some of the many financial and Johnson’s 133rd year was extraordinary. strategic achievements that were made possible by the commitment of our more than • We delivered strong operational revenue and 132,000 Johnson & Johnson colleagues, who adjusted operational earnings growth* that passionately lead the way in improving the health exceeded the financial performance goals we and well-being of people around the world. set for the Company at the start of 2019. • We again made record investments in research and development (R&D)—more than $11 billion across our Pharmaceutical, Medical Devices Propelled by our people, products, and and Consumer businesses—as we maintained a purpose, we look forward to the future relentless pursuit of innovation to develop vital with great confidence and optimism scientific breakthroughs. as we remain committed to leading • We proudly launched new transformational across the spectrum of healthcare. medicines for untreated and treatment-resistant diseases, while gaining approvals for new uses of many of our medicines already in the market. Through proactive leadership across our enterprise, we navigated a constant surge • We deployed approximately $7 billion, of unique and complex challenges, spanning primarily in transactions that fortify our dynamic global issues, shifting political commitment to digital surgery for a more climates, industry and competitive headwinds, personalized and elevated standard of and an ongoing litigious environment. healthcare, and that enhance our position in consumer skin health. As we have experienced for 133 years, we • And our teams around the world continued can be sure that 2020 will present a new set of working to address pressing public health opportunities and challenges. -

Submission to the Senate Community Affairs Committee: Number of Women in Australia Who Have Had Transvaginal Mesh Implants and Related Matters
Johnson & Johnson Medical Pty Ltd Submission to the Senate Community Affairs Committee: Number of women in Australia who have had transvaginal mesh implants and related matters May 2017 1 Our Credo We believe our first responsibility is to the doctors, nurses and patients, to mothers and fathers and all others who use our products and services. In meeting their needs everything we do must be of high quality. We must constantly strive to reduce our costs in order to maintain reasonable prices. Customers’ orders must be serviced promptly and accurately. Our suppliers and distributors must have an opportunity to make a fair profit. We are responsible to our employees, the men and women who work with us throughout the world. Everyone must be considered as an individual. We must respect their dignity and recognise their merit. They must have a sense of security in their jobs. Compensation must be fair and adequate, and working conditions clean, orderly and safe. We must be mindful of ways to help our employees fulfil their family responsibilities. Employees must feel free to make suggestions and complaints. There must be equal opportunity for employment, development and advancement for those qualified. We must provide competent management, and their actions must be just and ethical. We are responsible to the communities in which we live and work and to the world community as well. We must be good citizens - support good works and charities and bear our fair share of taxes. We must encourage civic improvements and better health and education. We must maintain in good order the property we are privileged to use, protecting the environment and natural resources. -

2019 GLOBAL WINNER Videos Produced By
2019 GLOBAL WINNER videos produced by Differences make a difference Professional and patient education SYMTUZA® consumer launch campaign My virtual surgery platform- ETHIBOND® & PROLENE® Janssen Biologics BV Ethicon LLC North America, United States North American, United States Pharmaceutical Medical Devices VIDEO LINK VIDEO LINK 2019 REGIONAL WINNER videos produced by Data inspired creativity Differences make a Purpose in action NICORETTE® inhalator travel difference JOHNSON’S® Baby – the right campaign Project CAST, embedding start our D&I commitments Johnson & Johnson (Ireland) in external brand Johnson & Johnson Middle East Limited FZ-LLC campaigns United Kingdom United Arab Emirates Johnson & Johnson Limited Consumer Consumer United Kingdom Consumer VIDEO LINK VIDEO LINK To view the videos, must be J&J on the view J&J network. employee computer To VIDEO LINK ! 1 Total brand experience Uncommon courage Fail forward The JOHNSON’s Healthy Skin TYZINE® in Russia: Janssen commitment to for Healthier Babies Successful Glocalization Rheumatology Guerrilla of «Headcold» IMC campaign Johnson & Johnson (Proprietary) J&J LLC Russia Janssen - Cilag South Africa Russia United Kingdom Consumer Consumer Pharmaceutical VIDEO LINK VIDEO LINK VIDEO LINK Healthcare provider Total brand experience Value through partnerships marketing LISTERINE® READY! TABS® American Red Cross Partnership Differentiating STELARA - a US Launch - disaster ready means always patient focused approach ready - BAND-AID® Brand Johnson & Johnson Consumer Adhesive Bandages & TYLENOL® Janssen - Cilag Inc. Johnson & Johnson Consumer Inc. Italy United States United States Pharmaceutical Consumer Consumer VIDEO LINK VIDEO LINK VIDEO LINK Uncommon courage ECLIPSE: TREMFYA vs. Cosentyx Head-to-Head Clinical Trial Janssen Biologics BV United States Pharmaceutical VIDEO LINK To view the videos, must be J&J on the view J&J network. -

Prag 3 a Ofteno Fco X 5 Ml Solucion Oftalmica 3
ANEXO No.2 INVITACIÓN ABIERTA No. 020-2008 LISTADO DE PRECIOS SUMINISTRO DE MEDICAMENTOS PERSONAL ACTIVO, PENSIONADO Y BENEFICAIRIOS DEL PLAN COMPLEMENTARIO DE SALUD 2009-2010 PRAG 3 A OFTENO FCO X 5 ML SOLUCION OFTALMICA 3 FRASCO/5 G/ML 0 59.308 BAGO 6-COPIN CJA X 12 COMPRIMIDOS 0 19.071 BAGO 6-COPIN GOTAS FSCO X 10 MILI 0 23.942 BAGO 6-COPIN X 3 AMPOLLAS 0 18.077 HUMA ABACAVIR 300 MG X 60 TAB 0 512.821 BRIS ABILIFY (ARIPIPRAZOL) 15 MG X 10 TAB TABLETA 15 MG 0 322.687 SCAN ABRILAR JARABE X 100 ML 0 27.436 SKIN ABSORB-K X 30 GR CREMA 30 G 16 57.115 REYM ABSORCAL-D X 30 TABLETAS 0 16.442 REYM ABSORCAL-D X 60 TABLETAS 0 19.667 PRCO ACAR-KLEAN X 400 ML 0 18.974 PROD ACCU CHECK ACTIVE X 50 GTS ACCU CHECK X 25 0 159.919 PROD ACCU-CHEK ACTIVE KIT 16 36.839 PROD ACCU-CHEK ACTIVE STRIPS X 25 0 94.534 PROD ACCU-CHEK ACTIVE STRIPS X 50 0 155.263 PROD ACCU-CHEK ACTIVE STRIP X 10 0 54.656 PROD ACCU CHEK ACTIVE X 50 2 CJS GTS KIT+ LANCE X 25 0 310.526 PROD ACCU-CHEK ACTIVE X 50 GTS AC ACTIVE KIT NEW TABLETA 0 142.748 PROD ACCU-CHEK ACTIV X50 PG2 GT1X25 0 267.16 PROD ACCU-CHEK GO KIT SD 16 161.736 PROD ACCU-CHEK GO X 25 0 98.178 PROD ACCU-CHEK GO X 50 0 155.87 PROD ACCU-CHEK GO X 50 GTS KIT 16 271.795 PROD ACCU-CHEK KIT GTS KIT MANICURE SD 16 83.484 PROD ACCU-CHEK LANCETAS + ACCU-CHEK X 50 TIRAS GTS KIT SD 0 174.089 PROD ACCU-CHEK LANCET X 25 UNDS 16 18.826 PROD ACCU-CHEK MULTICLIX X 24 16 25.641 PROD ACCU-CHEK PERFORMA KIT 16 89.891 PROD ACCU-CHEK PERFORMA TIRAS REACTIVAS X 50 0 164.53 PROD ACCU CHEK SOFTCLIX 2 CAJS X 25 GTS PUNCIONADOR 16 37.652 -

Sow Attachment 1: General Commodities and General Medical Surgical Supplies List
SOW ATTACHMENT 1: GENERAL COMMODITIES AND GENERAL MEDICAL SURGICAL SUPPLIES LIST GENERAL COMMODITIES LIST Air Compressor, Portable, 1 Gallon Backboard, 16" Wide, Orange Bag, Sand Bag, Trash (Regular 20-40 Gal) Battery, Alkaline, Size AA (1.5 Volt) Battery, Alkaline, Size AAA (1.5 Volt) Battery, Alkaline, Size C (1.5 Volt) Battery, Booster Cables 25' Binder, 3 Ring, 1-1/2" (for Cache Inventory Book) Bladder, Cap Assembly Bladder, Gray Water, 500 Gal (Grey Color) Bladder, Gray Water, Adaptor Bladder, Gray Water, In-Line Valve, 1¼" Bladder, Potable Water, 500 Gal (Blue Color) Bladder, Potable Water, In-Line Valve, 1" Body Bags / Tarps, Container, Rubbermaid, 36" Broom Cable Ties, Black UV Resistant, 11¼" Cache Inventory Sheets (in Cache Inventory Book) Can, Fuel, 5 Gal, Plastic, Diesel (Yellow) Can, Fuel, 5 Gal, Plastic, Gasoline (Red) Can, Fuel, 5-Gal, Plastic, GI Type (Green) Can, Fuel, 5-Gal, Plastic, GI Type (Green), Filler Spout Can, Water, 5 Gal, Plastic Chairs, Folding, Metal (no fabric) Cleaning/Disinfectant, 1 Gallon Cleanser, Antibacterial Hand, w/pump, 7-10 oz Cones, Traffic Container, Rubbermaid, Spare Latch Cot, Aluminum Detector, Carbon Monoxide & Smoke Discharge Hose, Gray Water (Black), 15' Discharge Hose, Gray Water (Black), 25' Discharge Hose, Potable Water, Cold (Blue), 15' Discharge Hose, Potable Water, Cold (Blue), 25' Discharge Hose, Potable Water, Hot (Red), 15' Discharge Hose, Potable Water, Hot (Red), 25' Discharge Hose, Potable Water, Hot (Red), 72" Door, Hard Door For Operating Room Drain Pan, 15 Quart Dust Pan -

FORM 10-Q Johnson & Johnson
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-Q ☑ Quarterly Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 for the quarterly period ended September 27, 2020 or Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 ☐ for the transition period from to Commission file number 1-3215 Johnson & Johnson (Exact name of registrant as specified in its charter) New Jersey 22-1024240 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification No.) One Johnson & Johnson Plaza New Brunswick, New Jersey 08933 (Address of principal executive offices) Registrant’s telephone number, including area code (732) 524-0400 Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☑ Yes ☐ No Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☑ Yes ☐ No Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and "emerging growth company" in Rule 12b-2 of the Exchange Act. -

Item ID Item Description
Item ID Item Description 0NE TOUCH ULTRASMAR ONE TOUCH ULTRA SMART MACHINE 10ML 10ML SYRINGES-NEED x 100 18G 18G CANULAR 1ML 1ML SYRINGES (INSULIN) 100 I.U 1ML SYRINGE 40I.U 1ML SYRINGE 40 I.U 20ML 20ML SYRINE 21G 21G NEEDLES x 1 1/2" X100 23G 23G NEEDLES x 1 1/4" X 100 25G 25G NEEDLES x 100 2ML 2ML SYRINGE 2ML SYRINGE B.D 2ML SYRINGE B.D 30 DAYS 30 DAYS BEAUTY SECRET 4711 COLONGE 4711 COLOGNE SPRAY 90ML 50ML SYRINGE 50ML SYRINGE 5ML SYRINGE 5ML SYRINGE 5ML SYRINGE BD 5ML SYRINGE BD 5ML SYRINGE INJ 5ML SYRINGE INJK/B-BRAUN/KDJ 7KEY HERBAL 7KEYS HERBAL MIXTURE 200ML ABAKTAL 400MG ABAKTAL 400MG x 100 ABAKTAL 5ML ABAKTAL 5ML IV x 10 ABF -3 CREAM ABF-3 CREAM 20G ABIDEC DROP 250 NIG ABIDEC NIG 250 MG NIG ABIDEC DROP 25ML ABIDEC DROP 25ML NATURAL FLAVO ABIDEC SYR ABIDEC M/VIT /OMEGA SYR 150ML ABITREN ABITREN x 100 ABITREN 10 ABITREN TABS x 10 ABONIKI BALM ABONIKI BALM 25GM ABSOLUTELY ABSOLUTELY FLAT FAST ACC-CHECK ACTIV ACC-CHECK ACTIVE MECHINE ACC-CHECK ACTIVE ACC-CHECK ACTIVE x 50 ACC-CHECK ACTIVE GRE ACC-CHEK ACTIVE MACHIN GREEN ACC-CHECK ADV II ACC-CHECK ADV II STRIPS X 50 ACC-CHECK ADVANTAGE ACC-CHECK ADV STRIPS X 50 ACC-CHECK AVIVA ACC-CHEK AVIVA STRIP X 50 ACC-CHECK GO STRIP ACC-CHECK GO STRIP X50 ACC-CHECK LANCENT ACC-CHECK LANCENT X200STRIPS ACC-CHECK LARGE ACC-CHECK ADVA MECHINE ACC-CHECK MECHINE ACC-CHECK MECHINE ADVANTAGE ACC-CHECK STRIP ACCU-CHECK COMFORT STRIPX50 ACC-CHEK ACTIVE X25 ACC-CHEK ACTIVE STRIP X25 ACC-CHEK AVIVA MACHI ACC-CHEK AVIVA MACHINE ACC-CHEK AVIVA NANO ACC-CHEK AVIVA NANO MACHINE ACC-CHEK COMPACT ACC-CHEK COMPACT -

Number Decscription Charge 380000 ABO
Charleston Surgical Hospital Number Decscription Charge 380000 ABO BLOOD TYPING $ 16.07 380008 ANA $ 90.59 380012 ANTI MICROSOMAL ANTIBODIE $ 81.11 380013 ANTIBODY SCREEN $ 143.96 380016 BARTONELLA $ 54.75 380017 BETA HCG $ 41.87 380020 BUN $ 22.01 380022 CALCIUM $ 28.72 380023 CBC-DIFF $ 36.09 380026 CKMB $ 55.76 380027 CLOSTRIDIUM CULTURE $ 110.02 380029 URINALYSIS $ 17.63 380032 C-REACTIVE PROTEIN $ 28.85 380033 CROSSMATCH ADDL LEVEL $ 41.69 380035 CULTURE-ANAEROBIC $ 52.73 380037 CYTOLOGY INTERP.& REPORT NEEDLE ASPI $ 232.32 380038 DECALCIFICATION $ 26.06 380042 DIRECT ANTIGEN FOR CULT $ 56.82 380044 DIRECT FUNGAL SMEAR $ 32.01 380048 ELECTROLYTES $ 32.87 380050 EXT STUDY OVER 5 SLIDES $ 33.42 380051 FACTOR VIII $ 236.75 380052 FERRITIN $ 102.14 380053 FIRST CROSS MATCH $ 41.69 380055 FLOW CYTOMETRY 9 - 15 $ 162.94 380056 FLOW CYTOMETRY EACH MARK $ 48.63 380058 FOLATE LEVEL $ 81.93 380059 FREE T4 $ 50.26 380061 FROZEN SECTION/SINGL SPEC $ 129.69 380063 FUNGUS CULTURE, OTHER $ 46.82 380064 GLUCOSE BY GLUCOMETER $ 13.06 380065 GLUCOSE FAST P C OR RANDM $ 21.87 380066 GRAM SMEAR $ 32.01 380068 HBSAG $ 51.92 380070 HELICOBACTOR PYLORI ANTI $ 80.90 380071 HEMATOCRIT $ 13.19 380072 HEMOGLOBIN $ 13.19 380074 HEPATIC PANEL $ 45.54 380079 HGB A1C $ 72.71 380080 HIV $ 45.10 380083 IDENTIFICATION PANEL $ 45.06 380084 IMMUNOPEROXIDASE STAIN $ 211.23 380085 INHALENT ALLERGEN $ 29.09 380089 IRON $ 36.12 380091 LEUKOCYTE-FLOW CYTOMETRY $ 126.52 380092 LIPID PANEL I $ 82.08 380094 MAGNESIUM $ 37.35 380095 METABOLIC-BASIC $ 47.14 380096 METABOLIC-COMPREHENSIVE -

Mad River Community Hospital Charge Master
Mad River Community Hospital Charge Master Item Descr IPprice 1000517 INFUSE DRUG INITIAL 2ND SITE $ 626 1000525 INFUSE DRUG INITIAL 3RD SITE $ 626 1044804 CRITICAL CARE 1 (30>74 MIN) $ 7,993 1044820 CRITICAL ADD 30 MIN $ 1,569 1044986 ER LEVEL TRIAGE $ 109 1045009 ER LEVEL I $ 505 1045017 ER LEVEL II $ 1,011 1045025 ER LEVEL III $ 1,740 1045033 ER LEVEL IV $ 2,889 1045041 ER LEVEL V $ 4,427 1045141 CODE TRAUMA W/ PRE-HOSPITAL NOTIFICATION $ 8,000 1045143 CODE TRAUMA W/O NOTIFICATION (1) $ 8,000 1045144 GASTROSTOMY TUBE CHANGE W/O U/S $ 1,071 1045145 EXCISION OF THROMBOSED EXT HEMORRHOID $ 4,370 1045146 REDUCE FX DISTAL HUM SUPRA W/O MANIP $ 1,035 1045147 REDUCE FX DISTAL HUM SUPRA W/MANIP $ 5,253 1045148 REDUCTION DISL IP JOINT FINGER W/O ANES $ 1,003 1045150 REDUCTION OF HIP FRACTURE W/O GEN ANES $ 1,003 1045152 INSERT SUPRAPUBIC CATHETER $ 7,913 1045223 INJ IMMUN 1 VACCINE $ 135 1045231 INJ IMMUN ADD VACCINE $ 135 1045241 TRAUMA ALERT W/ PRE-HOSPITAL NOTIF $ 8,000 1045243 TRAUMA ALERT W/O NOTIFICATION (1) $ 8,000 1045256 HYDRATION INITIAL 31-60 MIN $ 689 1045314 HYDRATE EACH ADD HOUR>31MIN $ 689 1045330 INFUSE DRUG EACH ADD HOUR >31MIN $ 466 1045371 INFUSE DRUG INITIAL 1ST 16-60 MIN $ 689 1045413 INTRA-ARTERIAL INJECTION OR INFUSION $ 274 1045415 THORACOSTOMY $ 4,587 1045417 I&D PILONIDAL CYST $ 2,665 1045419 I&D BARTHOLIN CYST $ 750 1045421 INFUSE DRUG CONCURR $ 466 1045439 IV PUSH INITIAL DRUG $ 274 1045454 IV PUSH EACH NEW DRUG $ 274 1045488 IV PUSH SAME DRUG>30MIN $ 274 1045702 CPR $ 988 1045744 INJECTION IM/SQ EACH INJECTION $