H FC BC IBC (Page 3)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stomachache Relief
Stomachache Relief Abdominal pain has many causes. Only rarely is the cause serious. The general information section of Pediatric Planet has more information about when to be concerned. If your child’s physician has determined that there is not a serious cause of the abdominal pain there are a number of over-the-counter medications that may be helpful. The key to getting benefit is choosing the right medication for the job. Having some opinion from your child’s doctor regarding the probable cause of the discomfort is a good start. Constipation may cause abdominal pain when gas is trapped behind blockages of hard stool. In this case see the constipation relief section of the medicine cabinet for more information. Abdominal pain may also accompany lactose intolerance. If your child is lactose intolerant avoidance of milk products and use of supplemental lactase (Dairy-ease, Lactaid), when necessary, will be helpful. Active ingredients: Aluminum Hydroxide (solitary active ingredient in some Amphojel, one of several active ingredients in some Gaviscon, Maalox, and Mylanta products). Aluminum hydroxide is an effective neutralizer of stomach acid although it does not work as quickly as calcium carbonate. It has a more prolonged effect, however, and does not cause bloating. Only small amounts of aluminum are absorbed into the blood stream, those with normal kidneys will excrete the aluminum in the urine. Unlike calcium, however, aluminum has no useful purpose to the body. In those with kidney problems aluminum may build up in the body causing osteoporosis, muscle weakness, and brain injury. There is some evidence linking aluminum to Alzheimer’s. -

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

Personalized Medicine for Reconstruction of Critical-Size Bone
www.nature.com/npjregenmed ARTICLE OPEN Personalized medicine for reconstruction of critical-size bone defects – a translational approach with customizable vascularized bone tissue ✉ Annika Kengelbach-Weigand 1 , Carolina Thielen 1, Tobias Bäuerle2, Rebekka Götzl 1,5, Thomas Gerber3, Carolin Körner4, Justus P. Beier1,5, Raymund E. Horch 1 and Anja M. Boos1,5 Tissue engineering principles allow the generation of functional tissues for biomedical applications. Reconstruction of large-scale bone defects with tissue-engineered bone has still not entered the clinical routine. In the present study, a bone substitute in combination with mesenchymal stem cells (MSC) and endothelial progenitor cells (EPC) with or without growth factors BMP-2 and VEGF-A was prevascularized by an arteriovenous (AV) loop and transplanted into a critical-size tibia defect in the sheep model. With 3D imaging and immunohistochemistry, we could show that this approach is a feasible and simple alternative to the current clinical therapeutic option. This study serves as proof of concept for using large-scale transplantable, vascularized, and customizable bone, generated in a living organism for the reconstruction of load-bearing bone defects, individually tailored to the patient’s needs. With this approach in personalized medicine for the reconstruction of critical-size bone defects, regeneration of parts of the human body will become possible in the near future. npj Regenerative Medicine (2021) 6:49 ; https://doi.org/10.1038/s41536-021-00158-8 1234567890():,; INTRODUCTION vascular networks consisting of endothelial cells can be created Therapeutic options for bone defects that cannot heal sponta- directly within tissue replacement materials7. Vascularization may neously, the so-called critical-size bone defects, are still limited be further supported by the addition of endothelial cells and and often associated with a great social burden. -

Medications in Pregnancy & Lactation.Xlsx
Commonly Used Medications in Pregnancy and Lactation Breastfeeding Medications: Indication & Side Notes Comments Acne: Over the counter acne medications are low risk. Acne ● Benzoyl Peroxide products Acne Low risk Clindamycin topical Acne Low risk Erythromycin topical Acne Low risk *Finacea topical Acne Not recommended Proactiv Acne Low risk Salicylic Acid products Acne Low risk Allergies: Actifed (after 13 weeks) Nasal Congestion, Allergies Low risk Afrin Nasal Spray (only for 3 days) Nasal Congestion Low risk Low risk (may ↓ milk Alavert (Loratadine) Allergies supply) Low risk (may ↓ milk Benadryl (Diphenhydramine) Allergies & Nasal Congestion supply) Low risk (may ↓ milk Clarinex Allergies supply) Low risk (may ↓ milk Claritin (Loratadine) Allergies supply) Low risk (may ↓ milk Claritin D (after 13 weeks) Allergies & Nasal Congestion supply) Low risk (may ↓ milk Chlor-Trimeton Allergies supply) Flonase Rhinitis, Seasonal Allergies Low risk Phenylephrine (after 13 weeks) Nasal Congestion use caution Ocean’s Nasal Spray Allergies & Nasal Congestion Low risk Low risk (may ↓ milk Sudafed (Pseudoephedrine) (after 13 weeks) Nasal Congestion supply) Low risk (may ↓ milk Tavist (Clemastine) Allergies supply) ● Please Contact Your Pediatrician concerning use in breast feeding. * Prescription medications Low risk (may ↓ milk Zyrtec Allergies supply) Antibiotics: *Amoxicillin Infection Low risk *Ampicillin Infection Low risk *Augmentin Infection Low risk *Keflex (Cephalexin) Infection Low risk *Cefuroxime Infection Low risk *Duricef (Cefadroxil) -

Approved Prenatal Medications Pain Medications • Tylenol
Approved Prenatal Medications Pain Medications Tylenol (acetaminophen) for minor aches and pains, headaches. (Do not use: Aspirin, Motrin, Advil, Aleve, Ibuprofen.) Coughs/Colds Robitussin (Cough) Robitussin DM (non-productive cough) DO NOT USE TILL OVER 12 WEEKS Secrets and Vicks Throat Lozenges Mucinex Sore Throat Chloraseptic spray Saline Gargle Sucrets and Vicks Throat Lozenges Antihistamines/Allergies Zyrtec Claritin Benadryl Dimetapp Insomnia Benadryl Unison Hemorrhoids Preparation H Tucks Anusol Diarrhea Imodium (1-2 doses- if it persists please notify the office) BRAT diet (bananas, rice, applesauce, toast) Lice RID (only!) DO NOT USE Kwell Itching Benadryl Calamine or Caladryl Lotion Hydrocortisone cream Heartburn, Indigestion, Gas Tums Gas-X Mylanta Pepcid Maalox Zantac *DO NOT USE PEPTO BISMOL- it contains aspirin Decongestants Sudafed Robitussin CF- Only if over 12 weeks Tavist D Ocean Mist Nasal Spray (saline solutions) Nausea Small Frequent Meals Ginger Ale Vitamin B6 Sea Bands Yeast Infections Monistat Mycolog Gyne-lotrimin Toothache Orajel May see dentists, have cavity filled using Novocain or lidocaine, have x-rays with double lead shield, may have antibiotics in the Penicillin family (penicillin, amoxicillin) Sweetners- all should be consumed in moderation with water being consumed more frequently Nutrisweet (aspartame) Equal (aspartame) Splenda (sucralose) Sweet’n Low (saccharin) *note avoid aspartame if you have phenylketonuria (PKU) Constipation Colace Fibercon Citrucel Senokot Metamucil Milk of Magnesia Fiberall Miralax Eczema Hydrocortisone Cream Medications to AVOID Accurate Lithium Paxil Ciprofloxacin Tetracycline Coumadin Other Chemicals to AVOID Cigarettes Alcohol Recreational Drugs: marijuana, cocaine, ecstasy, heroin . -
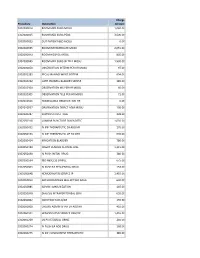
Procedure Description Charge Amount 0302000014 ROOM MED
Charge Procedure Description Amount 0302000014 ROOM MED SURG MOSU 1,040.00 0302000015 ROOM MED SURG PEDS 3,500.00 0302000033 OUT PATIENT BED MOSU 0.00 0302000035 ROOM INTERMEDIATE MOSU 2,055.00 0302000043 ROOM HOSPICE MOSU 805.00 0302000045 ROOM MED SURG W TELE MOSU 1,530.00 0302000050 OBSERVATION INTERM PER HR MOSU 97.00 0302002283 FECAL MANAGEMENT SYSTEM 694.00 0302010232 CATH INDWELL BLADDER SIMPLE 185.00 0302010304 OBSERVATION MS PER HR MOSU 60.00 0302010305 OBSERVATION TELE PER HR MOSU 71.00 0302010556 NONBILLABLE OBSERVATION HR 0.00 0302010557 OBSERVATION DIRECT ADM MOSU 130.00 0302020287 SUPPLIES CHEST TUBE 228.00 0302050318 LUMBAR PUNCTURE DIAGNOSTIC 1,035.00 0302050451 IV INF THERAPEUTIC EA ADD HR 170.00 0302050453 IV INF THERAPEUTIC UP TO 1HR 635.00 0302050454 IRRIGATION BLADDER 780.00 0302050480 INSERT VENOUS CENTRAL LINE 1,315.00 0302050490 IV PUSH INITIAL DRUG 380.00 0302050534 I&D ABSCESS SIMPLE 675.00 0302050603 IV PUSH EA SEQUENTIAL DRUG 153.00 0302050648 HEMODIALYSIS SERVICE IP 1,455.00 0302050813 ARTHROCENTESIS MAJ JNT WO IMAG 630.00 0302050885 ADMIN IMMUNIZATION 145.00 0302050948 DIALYSIS INTRAPERITONEAL SERV 655.00 0302060002 INJECTION SUB-Q/IM 155.00 0302060008 CHEMO ADMIN IV INF EA ADD HR 410.00 0302060101 HEMODIALYSIS SERVICE OBS/OP 1,455.00 0302060269 US PV RESIDUAL URINE 240.00 0302060274 IV PUSH EA ADD DRUG 168.00 0302060275 IV INF CONCURRENT THERAPEUTIC 385.00 0302060276 IV INF SEQUENTIAL THER UP TO 1 191.00 0302060293 INSERT STRAIGHT CATH THERAPEUT 185.00 0302060372 CHEMO ADMIN IV INF SEQ 1 HR 525.00 0302060373 -

Nevada Medicaid Formulary
Nevada Medicaid-Approved Preferred Drug List Effective August 15, 2021 Legend In each class, drugs are listed alphabetically by either brand name or generic name. Brand name drug: Uppercase in bold type Generic drug: Lowercase in plain type AL: Age Limit Restrictions DO: Dose Optimization Program GR: Gender Restriction OTC: Over the counter medication available with a prescription. (Prescribers please indicate OTC on the prescription) PA: Prior authorization is required. Prior authorization is the process of obtaining approval of benefits before certain prescriptions are filled. QL: Quantity limits; certain prescription medications have specific quantity limits per prescription or per month. SP: Specialty Pharmacy ST: Step therapy is required. You may need to use one medication before benefits for the use of another medication can be authorized. Drug Name Reference Notes *ADHD/ANTI-NARCOLEPSY/ANTI- OBESITY/ANOREXIANTS* *ADHD AGENT - SELECTIVE ALPHA ADRENERGIC AGONISTS*** clonidine hcl er oral tablet extended release Kapvay AL; QL 12 hour *ADHD AGENT - SELECTIVE NOREPINEPHRINE REUPTAKE INHIBITOR*** atomoxetine hcl oral capsule Strattera DO; AL; QL *AMPHETAMINE MIXTURES*** amphetamine-dextroamphet er oral capsule extended release 24 hour 10 mg, 15 mg, 5 Adderall XR DO; AL; QL mg amphetamine-dextroamphet er oral capsule extended release 24 hour 20 mg, 25 mg, 30 Adderall XR AL; QL mg amphetamine-dextroamphetamine oral Adderall DO; AL; QL tablet 10 mg, 12.5 mg, 15 mg, 5 mg, 7.5 mg amphetamine-dextroamphetamine oral Adderall AL tablet 20 mg, -

1CC FL Over the Counter (OTC) Benefit: List of Covered Products 081814
ANTACIDS MAST CELL STABILIZER Maalox-generic tablets, liquid Nasalcrom spray-generic Mylanta DS-generic liquid MINERALS ANTIBIOTICS Citracal – generic (calcium citrate) - tablets Bacitracin ointment-generic Citracal + D – generic (calcium citrate + D) – tablets Clotrimazole – cream, vaginal cream/inserts-generic Magnesium oxide-generic Miconazole – cream, vaginal cream/inserts - generic Neutra-phos/K powder-generic Tolnaftate – cream, gel, solution, aerosol - generic Oscal 500 + Vit D – generic (calcium carbonate + D) - tablets ANTI-DIARRHEALS Tums Chew Tabs – generic (calcium carbonate) Imodium A-D-generic (loperamide) capsules Pepto-Bismol-generic (pink bismuth) liquid 262mg/15ml MOSQUITO REPELLENT OFF! Family Care ANTI-EMETIC OFF! Deep Woods Antivert-generic (meclizine) OFF! Active Cutter Skinsations ANTI-FLATULENTS Cutter Backwoods Gas-X chewables – generic simethicone 80mg Repel Insect Sportsmen Mylicon drops** – generic simethicone 40 mg/0.6ml Repel Sportsmen Max Formula ANTI-HISTAMINES NASAL DECONGESTANT Benadryl-generic (diphenhydramine)-capsules, liquid Sudafed-generic (pseudoephedrine)-tablets, liquid Chlor-Trimeton-generic (chlorpheniramine)-tablets, liquid NSAIDS Claritin - generic (loratadine) – tablets, syrup Ibuprofen-generic tablets, chewable, liquid, drops Claritin-D- generic (loratadine/ pseudoephedrine) - Naproxen – generic tablets tablets NUTRITIONAL SUPPLEMENTS ANTITUSSIVE Ensure Robitussin DM -generic (guaifenesin DM) syrup Pediasure COUGH SUPPRESSANT/DECONGESTANT OPHTHALMIC PREPARATIONS Triaminic AM, Night, soft -

Over-The-Counter Mail Order Program 1-866-768-8490 As a Superior
Over-the-Counter Mail Order Program 1-866-768-8490 As a Superior value-added service, STAR+PLUS and STAR Health members can get $30 in items every 3 months (90 days). STAR members can get $25 in items every 3 months (90 days). No prescription is needed. To order, please call 1-866-768-8490. Have your Superior ID card ready when you call. Your order will be mailed to your home in 5-10 days. Please use these items only as directed. If you have questions about safe use of any of these items, talk to your doctor. Item Description Compare to: Price Item Description Compare to: Price Analgesics Eye Care 1 Ibuprofen 200mg tab Motrin IB $6 31 Tetrahydrozoline drops Visine $4 2 Naproxen sod 220mg tab Aleve $9 61 Lubricating eye drops Refresh Tears $7 3 Aspirin 325mg tab Bayer Aspirin $5 First Aid Creams/Ointments 4 Aspirin ec 325 mg tab Ecotrin $6 32 Calamine lotion Calamine Lotion $4 5 Aspirin ec 81 mg Halfprin $5 33 Hydrocortisone !5 cream Cort-Aid $4 6 Acetaminophen 500mg tab Tylenol Extra Str $6 34 Triple antibiotic ointment Neosporin $5 7 Mentholated ointment Ben Gay $6 60 Medicated lip balm Carmex $3 Antacids First Aid Supplies 8 Simethicone 80mg tab Mylanta Anti-Gas $6 35 Athletic bandage Ace Bandage $7 9 Calc carb 500mg chewable TUMS $6 36 Adhesive tape First-Aid Tape $3 10 Famotidine 10mg tab Pepcid AC $9 37 Band-aids Band-Aids $4 Antidiarrheals 38 Carbamide peroxide Debrox Drops $4 11 Loperamide 2mg cap Imodium $5 39 Gauze pads Gauze Pads $3 12 Bismuth mixture Pepto-Bismol $5 40 Cotton swab Q-Tips $4 Antifungals 41 Oral thermometer Thermometer -

Creative Director 561.714.1585 Andy Mathurin
Hello, I am: Looking for role as: Let’s Connect: Andy Mathurin Creative Director 561.714.1585 Associate Creative Director, [email protected] Brand Strategist New York What I do: PROFILE Brand Strategy Over 8 years leading branding and marketing concepts with career spanning 360 campaigns, broadcast, print, OOH, social, digital, video and experiential Identity Design across major worldwide brands. Energetic and a creative visionary offering Storytelling demonstrated expertise in all aspects of branding and strategy, with core focus Thought Leader on delivering business results. Team Builder Marketing WINS Project Management Successes have included winning several accounts for new business, resulting in agency being awarded Global Agency of the Year by AdAge & Adweek. Toolkit: AOR: Havas - Vascepa 2019, Havas - Alcon 2019, J3 - OGX 2018, UM - H&M 2017, UM - Sony 2017, BMW 2016, UM - Coca Cola 2016 Sketch XD Microsoft Office PROFESSIONAL EXPERIENCE Avocode 03.18 HAVAS | Manhattan, NY Photoshop Present Associate Creative Director Strategic partner, focusing on brand equity for healthcare and InDesign pharmaceutical brands. Responsible for new business and leading creative Illustration team/studio across all digital platforms (specializing in social). After Effects Brands include: Zicam, Alcon, GSK, NUCALA, TRELEGY, & ANORO. 09.14 Universal McCann Worldwide | Manhattan, NY 03.18 Personality: Associate Creative Director + Senior Art Director Hands-on leader in identity design. Champion participant in winning new Human business pitches. Guiding and advising clients on high-level executions. Confident Brands include: Sony Pictures, GoPro, McCormick, BMW... and more. Effective Communicator - Demonstrate strong leadership overseeing staff in the day-to-day projects Motivational Honest with Integrity - Deliver against demanding brand objectives and develop creative that Empathetic exceeds business needs, managing projects from concept through completion: timelines, budgets, schedules, etc. -

Lifescan Ethicon
LouiseLouise MehrotraMehrotra ViceVice PresidentPresident InvestorInvestor RelationsRelations ““SafeSafe HarborHarbor”” StatementStatement This presentation may contain “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could vary materially from the Company’s expectations and projections. Risks and uncertainties include general industry conditions and competition; economic conditions, such as interest rate and currency exchange rate fluctuations; technological advances and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approvals; domestic and foreign health care reforms and governmental laws and regulations; and trends toward health care cost containment. A further list and description of these risks, uncertainties and other factors can be found in Exhibit 99 to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2006. Copies of this Form 10-K, as well as subsequent filings, are available online at www.sec.gov, www.jnj.com or on request from the Company. The Company does not undertake to update any forward-looking statements as a result of new information or future events or developments. WilliamWilliam C.C. WeldonWeldon ChairmanChairman ofof thethe BoardBoard && ChiefChief ExecutiveExecutive OfficerOfficer TodayToday’’ss AgendaAgenda -

Bowling V. Johnson & Johnson, Et
Case 1:17-cv-03982-AJN Document 1 Filed 05/25/17 Page 1 of 21 IN THE UNITED STATES DISTRICT COURT FOR THE SOUTHERN DISTRICT OF NEW YORK SUZANNA BOWLING, individually and on behalf of all others similarly situated, CASE NO. Plaintiff, v. CLASS ACTION COMPLAINT JOHNSON & JOHNSON and McNEIL NUTRITIONALS, LLC, JURY TRIAL DEMANDED Defendants. Plaintiff Suzanna Bowling brings this action on behalf of herself and all others similarly situated against Defendants Johnson & Johnson and McNeil Nutritionals, LLC (“McNeil”) (collectively, “J&J” or “Defendants”). Plaintiff makes the following allegations pursuant to the investigation of her counsel and based upon information and belief, except as to the allegations specifically pertaining to herself, which are based on personal knowledge. NATURE OF THE ACTION 1. This is a class action lawsuit regarding Defendants’ false and misleading labeling of Benecol Regular and Light Spreads (together, “Benecol Spreads”), each of which uniformly claims that the product (i) contains “No Trans Fats” and “No Trans Fatty Acids,” and (ii) is generally recognized as safe for human consumption (the “Misrepresentations”). However, Benecol Spreads contain trans fat through the use of partially hydrogenated oils. Thus, the labels on Benecol Spreads are false and misleading. Case 1:17-cv-03982-AJN Document 1 Filed 05/25/17 Page 2 of 21 2. In June 2015, the FDA concluded that partially hydrogenated oils – the same oils found in Benecol Spreads – are not “generally recognized as safe” for use in human food due to “an increased risk of coronary heart disease by contributing to the buildup of plaque inside the arteries that may cause a heart attack.” Thus, Benecol Spreads are not generally recognized as safe for human consumption.