Johnson Medal Winners
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Learner Notification International Society for Heart & Lung
Learner Notification International Society for Heart & Lung Transplantation (ISHLT) 41st Annual Meeting & Scientific Sessions Virtual Experience April 24 – 28, 2021 Live Virtual Acknowledgement of Financial Commercial Support Abbott Medtronic United Therapeutics Acknowledgement of In-Kind Commercial Support No in-kind commercial support was received for this educational activity. Satisfactory Completion Learners must complete an evaluation form to receive a certificate of completion. Your chosen sessions must be attended in their entirety as partial credit of individual sessions is not available. If you are seeking continuing education credit for a specialty not listed below, it is your responsibility to contact your licensing/certification board to determine course eligibility for your licensing/certification requirement. Physicians (ACCME) The International Society for Heart and Lung Transplantation (ISHLT) is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. Credit Designation Statement - ISHLT designates this live virtual activity for a maximum of 32.00 AMA PRA Category 1 CreditsTM. Physicians should claim only the credit commensurate with the extent of their participation in the activity. Accreditation Statement In support of improving patient care, this activity has been planned and implemented by Amedco LLC and ISHLT. Amedco LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. Nurses (ANCC) - Credit Designation Statement - Amedco LLC designates this live virtual activity for a maximum of 32.00 ANCC contact hours. Pharmacists (ACPE) - Credit Designation Statement - Amedco LLC designates this live virtual activity for a maximum of 32.00 knowledge-based CPE contact hours. -

2019 Health for Humanity Report
2019 Health for Humanity Report Progress in Sustainability Report Summary Contents Message from Our Chairman and CEO 3 Sustainability Approach 4 2019 Year in Brief 5 Better Health for All: Tackling the World’s 6 Toughest Health Challenges Better Health for All: Access, Community 7 Health & Innovation Responsible Business Practices 8 Environmental Health 9 UNICEF, the Government of Vietnam and Johnson & Johnson are partnering on a national program to train more than 500 ethnic minority midwives in remote regions to provide effective maternal and child health interventions including early essential newborn care in village clinics and homes. Photo by Paul Bettings Front cover Volunteers, frontline health workers and government officials at the launch of the Umurinzi vaccination program in Rwanda. In October 2019, Johnson & Johnson committed to donating up to 700,000 regimens of Janssen’s investigational Ebola vaccine to support the Ebola outbreak response in Rwanda and the Democratic Republic of the Congo. Photos by Rwanda Ministry of Health 2019 Health2017 for Health Humanity for HumanityReport Summary Report 33 Message from Our Chairman and CEO Dear Johnson & Johnson Stakeholders, We know this mission will always be unfinished, and affirmed unequivocally that there is a fundamental that we will occasionally fall short. But that only serves connection between serving all stakeholders and 2019 was a year of profound change and great contrasts as motivation to move faster than we’ve ever moved generating sustainable, long-term value. around the globe. before in making bigger strides toward some of our most ambitious goals. And as we’ve detailed in this Report, we The demands for global healthcare and responsible We saw unprecedented innovation and encouraging have plenty of positive momentum worth recognizing. -

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

Personalized Medicine for Reconstruction of Critical-Size Bone
www.nature.com/npjregenmed ARTICLE OPEN Personalized medicine for reconstruction of critical-size bone defects – a translational approach with customizable vascularized bone tissue ✉ Annika Kengelbach-Weigand 1 , Carolina Thielen 1, Tobias Bäuerle2, Rebekka Götzl 1,5, Thomas Gerber3, Carolin Körner4, Justus P. Beier1,5, Raymund E. Horch 1 and Anja M. Boos1,5 Tissue engineering principles allow the generation of functional tissues for biomedical applications. Reconstruction of large-scale bone defects with tissue-engineered bone has still not entered the clinical routine. In the present study, a bone substitute in combination with mesenchymal stem cells (MSC) and endothelial progenitor cells (EPC) with or without growth factors BMP-2 and VEGF-A was prevascularized by an arteriovenous (AV) loop and transplanted into a critical-size tibia defect in the sheep model. With 3D imaging and immunohistochemistry, we could show that this approach is a feasible and simple alternative to the current clinical therapeutic option. This study serves as proof of concept for using large-scale transplantable, vascularized, and customizable bone, generated in a living organism for the reconstruction of load-bearing bone defects, individually tailored to the patient’s needs. With this approach in personalized medicine for the reconstruction of critical-size bone defects, regeneration of parts of the human body will become possible in the near future. npj Regenerative Medicine (2021) 6:49 ; https://doi.org/10.1038/s41536-021-00158-8 1234567890():,; INTRODUCTION vascular networks consisting of endothelial cells can be created Therapeutic options for bone defects that cannot heal sponta- directly within tissue replacement materials7. Vascularization may neously, the so-called critical-size bone defects, are still limited be further supported by the addition of endothelial cells and and often associated with a great social burden. -

HEALTH PROFESSIONAL CONSULTANT to a PHARMACEUTICAL COMPANY V JOHNSON & JOHNSON Nicorette Advertisement
CASE AUTH/2930/1/17 HEALTH PROFESSIONAL CONSULTANT TO A PHARMACEUTICAL COMPANY v JOHNSON & JOHNSON Nicorette advertisement A complaint was received in a private capacity that the implication was that the statement in from a health professional who stated that he/ question related to a feature of Nicorette, that the she worked as a consultant to a pharmaceutical product itself had incredible features and/or that company. health professionals would be doing something incredible by prescribing it. The implication was The complaint concerned an online advertisement misleading and exaggerated and breaches of the for Nicorette (nicotine) issued by Johnson & Code ruled. Johnson published in Pulse. The complainant stated at the time of submitting The complainant provided a screenshot of a the complaint that he/she was a health professional banner advertisement. It included ‘Nicorette. Do who worked as a consultant to Novartis. It had something incredible’. The complainant did not previously been decided, following consideration believe that the word ‘incredible’ was suitable. This by the then Code of Practice Committee and the information did not appear to be balanced and was ABPI Board of Management, that private complaints exaggerated. The claim was taken directly from from pharmaceutical company employees had material aimed at the general public and it appeared to be accepted. To avoid this becoming a means that Johnson & Johnson had not undertaken a of circumventing the normal procedures for sufficiently robust review when translating to intercompany complaints, the employing company promotion aimed at health professionals. would be named in the report. The complainant would be advised that this would happen and be The detailed response from Johnson & Johnson is given an opportunity to withdraw the complaint. -
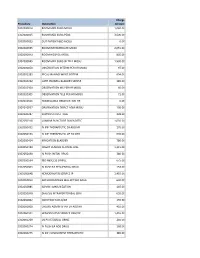
Procedure Description Charge Amount 0302000014 ROOM MED
Charge Procedure Description Amount 0302000014 ROOM MED SURG MOSU 1,040.00 0302000015 ROOM MED SURG PEDS 3,500.00 0302000033 OUT PATIENT BED MOSU 0.00 0302000035 ROOM INTERMEDIATE MOSU 2,055.00 0302000043 ROOM HOSPICE MOSU 805.00 0302000045 ROOM MED SURG W TELE MOSU 1,530.00 0302000050 OBSERVATION INTERM PER HR MOSU 97.00 0302002283 FECAL MANAGEMENT SYSTEM 694.00 0302010232 CATH INDWELL BLADDER SIMPLE 185.00 0302010304 OBSERVATION MS PER HR MOSU 60.00 0302010305 OBSERVATION TELE PER HR MOSU 71.00 0302010556 NONBILLABLE OBSERVATION HR 0.00 0302010557 OBSERVATION DIRECT ADM MOSU 130.00 0302020287 SUPPLIES CHEST TUBE 228.00 0302050318 LUMBAR PUNCTURE DIAGNOSTIC 1,035.00 0302050451 IV INF THERAPEUTIC EA ADD HR 170.00 0302050453 IV INF THERAPEUTIC UP TO 1HR 635.00 0302050454 IRRIGATION BLADDER 780.00 0302050480 INSERT VENOUS CENTRAL LINE 1,315.00 0302050490 IV PUSH INITIAL DRUG 380.00 0302050534 I&D ABSCESS SIMPLE 675.00 0302050603 IV PUSH EA SEQUENTIAL DRUG 153.00 0302050648 HEMODIALYSIS SERVICE IP 1,455.00 0302050813 ARTHROCENTESIS MAJ JNT WO IMAG 630.00 0302050885 ADMIN IMMUNIZATION 145.00 0302050948 DIALYSIS INTRAPERITONEAL SERV 655.00 0302060002 INJECTION SUB-Q/IM 155.00 0302060008 CHEMO ADMIN IV INF EA ADD HR 410.00 0302060101 HEMODIALYSIS SERVICE OBS/OP 1,455.00 0302060269 US PV RESIDUAL URINE 240.00 0302060274 IV PUSH EA ADD DRUG 168.00 0302060275 IV INF CONCURRENT THERAPEUTIC 385.00 0302060276 IV INF SEQUENTIAL THER UP TO 1 191.00 0302060293 INSERT STRAIGHT CATH THERAPEUT 185.00 0302060372 CHEMO ADMIN IV INF SEQ 1 HR 525.00 0302060373 -

Frequently Asked Questions
Frequently Asked Questions Why are these companies included on the "Do Test" list? The following companies manufacture products that are tested on animals at some stage of development. Those marked with a Ƈ are currently observing a moratorium on (i.e., current suspension of) animal testing. Please encourage them to announce a permanent ban. Listed in parentheses are examples of products manufactured by either the company listed or, if applicable, its parent company. For a complete listing of products manufactured by a company on this list, please visit the company's website or contact the company directly for more information. Companies on this list may manufacture individual lines of products that have not been tested on animals. They have not, however, eliminated tests on animals for their entire line of cosmetics and household products. What if a company isn't on either of PETA's lists? Some companies have refused to respond to specific questions about their testing practices. It appears likely that these companies do test on animals at some stage of product development, and their refusal to clarify their testing policies appears to be an attempt to mislead consumers. Other companies may be new. If you find a company not included on our lists, please share the company's contact information with PETA so that we can contact the company directly. Legend Ƈ The company is currently observing a moratorium on animal testing. 3M 3M Corporate 1-888-364-3577 www.solutions.3m.com Headquarters 3M Center St. Paul, Minnesota 55144-1000 Acuvue (Johnson & Johnson) Customer (800) 843-2020 www.acuvue.com/ Relations, D-QA 7500 Centurion Parkway Jacksonville, Florida 32256 Aim (Church & Dwight) P.O. -

Nicorette Invisipatch 25 Mg/16 H Transdermal Patch
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Nicorette invisipatch 25 mg/16 h transdermal patch Nicorette invisipatch 15 mg/16 h transdermal patch Nicorette invisipatch 10 mg/16 h transdermal patch 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each transdermal patch contains nicotine 1.75 mg/cm2. Nicorette invisipatch 25 mg/16 h, of 22.5 cm2 size contains nicotine 39.37 mg and releases nicotine 25 mg /16 hours Nicorette invisipatch 15 mg/16 h, of 13.5 cm2 size contains nicotine 23.62 mg and releases nicotine 15 mg /16 hours Nicorette invisipatch 10 mg/16 h, of 9.0 cm2 size contains nicotine 15.75 mg and releases nicotine 10 mg /16 hours For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Transdermal patch Beige, semi-transparent, rectangular patch with rounded edges and light-brown “Nicorette” printing, is placed on an easily removable layer coated with aluminium and silicon and is formed by nicotine layer and adhesive acrylate layer. 4. CLINICAL PARTICULARS 4.1. Therapeutic indication Nicorette invisipatch is to be used for the treatment of tobacco dependence in adults by relief of nicotine withdrawal symptoms, including cravings, during a quit attempt. Permanent cessation of tobacco use is the eventual objective. Nicorette invisipatch is indicated in adults. Nicorette invisipatch should preferably be used in conjunction with a behavioral support program. 4.2. Posology and method of administration Posology Subjects should stop smoking completely during the course of treatment with Nicorette invisipatch. Administration of nicotine should be stopped immediately if any symptoms of overdose listed in Section 4.9 occur. -

Over-The-Counter Mail Order Program 1-866-768-8490 As a Superior
Over-the-Counter Mail Order Program 1-866-768-8490 As a Superior value-added service, STAR+PLUS and STAR Health members can get $30 in items every 3 months (90 days). STAR members can get $25 in items every 3 months (90 days). No prescription is needed. To order, please call 1-866-768-8490. Have your Superior ID card ready when you call. Your order will be mailed to your home in 5-10 days. Please use these items only as directed. If you have questions about safe use of any of these items, talk to your doctor. Item Description Compare to: Price Item Description Compare to: Price Analgesics Eye Care 1 Ibuprofen 200mg tab Motrin IB $6 31 Tetrahydrozoline drops Visine $4 2 Naproxen sod 220mg tab Aleve $9 61 Lubricating eye drops Refresh Tears $7 3 Aspirin 325mg tab Bayer Aspirin $5 First Aid Creams/Ointments 4 Aspirin ec 325 mg tab Ecotrin $6 32 Calamine lotion Calamine Lotion $4 5 Aspirin ec 81 mg Halfprin $5 33 Hydrocortisone !5 cream Cort-Aid $4 6 Acetaminophen 500mg tab Tylenol Extra Str $6 34 Triple antibiotic ointment Neosporin $5 7 Mentholated ointment Ben Gay $6 60 Medicated lip balm Carmex $3 Antacids First Aid Supplies 8 Simethicone 80mg tab Mylanta Anti-Gas $6 35 Athletic bandage Ace Bandage $7 9 Calc carb 500mg chewable TUMS $6 36 Adhesive tape First-Aid Tape $3 10 Famotidine 10mg tab Pepcid AC $9 37 Band-aids Band-Aids $4 Antidiarrheals 38 Carbamide peroxide Debrox Drops $4 11 Loperamide 2mg cap Imodium $5 39 Gauze pads Gauze Pads $3 12 Bismuth mixture Pepto-Bismol $5 40 Cotton swab Q-Tips $4 Antifungals 41 Oral thermometer Thermometer -
![[J-52A-2014] in the Supreme Court of Pennsylvania Middle District](https://docslib.b-cdn.net/cover/1548/j-52a-2014-in-the-supreme-court-of-pennsylvania-middle-district-941548.webp)
[J-52A-2014] in the Supreme Court of Pennsylvania Middle District
[J-52A-2014] IN THE SUPREME COURT OF PENNSYLVANIA MIDDLE DISTRICT COMMONWEALTH OF PENNSYLVANIA : 84 MAP 2011 : : Appeal from the decision of the : Commonwealth Court (Opinion re Post- v. : Trial Motions of the Commonwealth and : Johnson & Johnson) dated 8/31/11 at No. : 212 MD 2004 TAP PHARMACEUTICAL PRODUCTS, : INC.; ABBOTT LABORATORIES; : ASTRAZENECA PLC; ASTRAZENECA, : HOLDINGS, INC.; ASTRAZENECA : PHARMACEUTICALS LP; : ASTRAZENECA LP; BAYER AG; BAYER : CORPORATION; SMITHKLINE : BEECHAM CORPORATION D/B/A : GLAXOSMITHKLINE; PFIZER, INC.; : PHARMACIA CORPORATION; : JOHNSON & JOHNSON; ALZA : CORPORATION; CENTROCOR, INC.; : ETHICON, INC.; JANSSEN : PHARMACEUTICAL PRODUCTS, L.P.; : MCNEIL-PPC, INC.; ORTHO BIOTECH, : INC.; ORTHO BIOTECH PRODUCTS; : L.P.; ORTHO-MCNEIL : PHARMACEUTICAL, INC.; AMGEN INC.; : IMMUNEX CORPORATION; BRISTOL- : MYERS SQUIBB COMPANY; BAXTER : INTERNATIONAL INC.; BAXTER : HEALTHCARE CORPORATION; : IMMUNO-U.S., INC.; AVENTIS : PHARMACEUTICALS, INC.; AVENTIS : BEHRING, L.L.C.; HOECHST MARION : ROUSSEL, INC., BOEHRINGER : INGELHEIM CORPORATION; : BOEHRINGER INGELHEIM : PHARMACEUTICALS, INC.; BEN VENUE : LABORATORIES; BEDFORD : LABORATORIES; ROXANE : LABORATORIES; SCHERING-PLOUGH : CORPORATION; WARRICK : PHARMACEUTICALS CORPORATION; : SCHERING SALES CORPORATION; : DEY, INC. : : DONNA A. BOSWELL, ESQ., ANN M. : VICKERY, ESQ., AND JOSEPH A. : YOUNG, ESQ., : Intervenors : : APPEAL OF: JOHNSON & JOHNSON, : ALZA CORPORATION, CENTOCOR, : INC., ETHICON, INC., JANSSEN : PHARMACEUTICAL PRODUCTS, L.P., : MCNEIL-PPC, INC., ORTHO BIOTECH, : INC., ORTHO BIOTECH PRODUCTS, : L.P., AND ORTHO-MCNEIL : PHARMACEUTICAL, INC. : (COLLECTIVELY, THE “JOHNSON & : JOHNSON DEFENDANTS”) : ORDER PER CURIAM DECIDED: June 16, 2014 AND NOW, this 16th day of June, 2014, the Order of the Commonwealth Court is VACATED, and the matter is remanded per the terms of Mr. Justice Baer’s responsive opinion in Commonwealth v. TAP Pharm. Prods., Inc., J-52B-2014, ___ A.3d ___ (Pa. -

Creative Director 561.714.1585 Andy Mathurin
Hello, I am: Looking for role as: Let’s Connect: Andy Mathurin Creative Director 561.714.1585 Associate Creative Director, [email protected] Brand Strategist New York What I do: PROFILE Brand Strategy Over 8 years leading branding and marketing concepts with career spanning 360 campaigns, broadcast, print, OOH, social, digital, video and experiential Identity Design across major worldwide brands. Energetic and a creative visionary offering Storytelling demonstrated expertise in all aspects of branding and strategy, with core focus Thought Leader on delivering business results. Team Builder Marketing WINS Project Management Successes have included winning several accounts for new business, resulting in agency being awarded Global Agency of the Year by AdAge & Adweek. Toolkit: AOR: Havas - Vascepa 2019, Havas - Alcon 2019, J3 - OGX 2018, UM - H&M 2017, UM - Sony 2017, BMW 2016, UM - Coca Cola 2016 Sketch XD Microsoft Office PROFESSIONAL EXPERIENCE Avocode 03.18 HAVAS | Manhattan, NY Photoshop Present Associate Creative Director Strategic partner, focusing on brand equity for healthcare and InDesign pharmaceutical brands. Responsible for new business and leading creative Illustration team/studio across all digital platforms (specializing in social). After Effects Brands include: Zicam, Alcon, GSK, NUCALA, TRELEGY, & ANORO. 09.14 Universal McCann Worldwide | Manhattan, NY 03.18 Personality: Associate Creative Director + Senior Art Director Hands-on leader in identity design. Champion participant in winning new Human business pitches. Guiding and advising clients on high-level executions. Confident Brands include: Sony Pictures, GoPro, McCormick, BMW... and more. Effective Communicator - Demonstrate strong leadership overseeing staff in the day-to-day projects Motivational Honest with Integrity - Deliver against demanding brand objectives and develop creative that Empathetic exceeds business needs, managing projects from concept through completion: timelines, budgets, schedules, etc. -

Ethics and Social Responsibility
Fundamentals of Business, Second Edition Chapter 4 Ethics and Social Responsibility Content for this chapter was adapted from the Saylor Foundation’s http://www.saylor.org/site/textbooks/Exploring%20Business.docx by Virginia Tech under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License. The Saylor Foundation previously adapted this work under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License without attribution as requested by the work’s original creator or licensee. If you redistribute any part of this work, you must retain on every digital or print page view the following attribution: Download this book for free at: http://hdl.handle.net/10919/84848 Lead: Stephen J. Skripak Contributors: Anastasia Cortes, Gary Walton, Anita Walz Digital and Print Production: Corinne Guimont with Robert Browder Alternative Text and Accessibility: Stephanie Edwards, Christa Miller, and Corinne Guimont Selected graphics: Brian Craig Cover design: Trevor Finney Student Reviewers: Jonathan De Pena, Nina Lindsay, Sachi Soni Project Manager/Editor: Anita Walz This chapter is licensed with a Creative Commons Attribution-Noncommercial-Sharealike 3.0 License. Download this book for free at: http://hdl.handle.net/10919/84848 VT Publishing, a division of the University Libraries at Virginia Tech August 2018 4. Chapter 4 Ethics and Social Responsibility Learning Objectives 1. Define business ethics and explain what it means to act ethically in business. 2. Explain why we study business ethics. 3. Identify ethical issues that you might face in business, such as insider trading, conflicts of interest, and bribery, and explain rationalizations for unethical behavior. 4. Identify steps you can take to maintain your honesty and integrity in a business environment.