A Characterization of Tonality Via the Rigidity of Chords
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unified Music Theories for General Equal-Temperament Systems
Unified Music Theories for General Equal-Temperament Systems Brandon Tingyeh Wu Research Assistant, Research Center for Information Technology Innovation, Academia Sinica, Taipei, Taiwan ABSTRACT Why are white and black piano keys in an octave arranged as they are today? This article examines the relations between abstract algebra and key signature, scales, degrees, and keyboard configurations in general equal-temperament systems. Without confining the study to the twelve-tone equal-temperament (12-TET) system, we propose a set of basic axioms based on musical observations. The axioms may lead to scales that are reasonable both mathematically and musically in any equal- temperament system. We reexamine the mathematical understandings and interpretations of ideas in classical music theory, such as the circle of fifths, enharmonic equivalent, degrees such as the dominant and the subdominant, and the leading tone, and endow them with meaning outside of the 12-TET system. In the process of deriving scales, we create various kinds of sequences to describe facts in music theory, and we name these sequences systematically and unambiguously with the aim to facilitate future research. - 1 - 1. INTRODUCTION Keyboard configuration and combinatorics The concept of key signatures is based on keyboard-like instruments, such as the piano. If all twelve keys in an octave were white, accidentals and key signatures would be meaningless. Therefore, the arrangement of black and white keys is of crucial importance, and keyboard configuration directly affects scales, degrees, key signatures, and even music theory. To debate the key configuration of the twelve- tone equal-temperament (12-TET) system is of little value because the piano keyboard arrangement is considered the foundation of almost all classical music theories. -

Mto.99.5.2.Soderberg
Volume 5, Number 2, March 1999 Copyright © 1999 Society for Music Theory Stephen Soderberg REFERENCE: MTO Vol. 4, No. 3 KEYWORDS: John Clough, Jack Douthett, David Lewin, maximally even set, microtonal music, hyperdiatonic system, Riemann system ABSTRACT: This article is a continuation of White Note Fantasy, Part I, published in MTO Vol. 4, No. 3 (May 1998). Part II combines some of the work done by others on three fundamental aspects of diatonic systems: underlying scale structure, harmonic structure, and basic voice leading. This synthesis allows the recognition of select hyperdiatonic systems which, while lacking some of the simple and direct character of the usual (“historical”) diatonic system, possess a richness and complexity which have yet to be fully exploited. II. A Few Hypertonal Variations 5. Necklaces 6. The usual diatonic as a model for an abstract diatonic system 7. An abstract diatonic system 8. Riemann non-diatonic systems 9. Extension of David Lewin’s Riemann systems References 5. Necklaces [5.1] Consider a symmetric interval string of cardinality n = c+d which contains c interval i’s and d interval j’s. If the i’s and j’s in such a string are additionally spaced as evenly apart from one another as possible, we will call this string a “necklace.” If s is a string in Cn and p is an element of Cn, then the structure ps is a “necklace set.” Thus <iijiij>, <ijijij>, and <ijjijjjijjj> are necklaces, but <iijjii>, <jiijiij>, and <iijj>, while symmetric, are not necklaces. i and j may also be equal, so strings of the 1 of 18 form <iii...> are valid necklaces. -
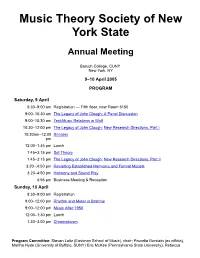
Program and Abstracts for 2005 Meeting
Music Theory Society of New York State Annual Meeting Baruch College, CUNY New York, NY 9–10 April 2005 PROGRAM Saturday, 9 April 8:30–9:00 am Registration — Fifth floor, near Room 5150 9:00–10:30 am The Legacy of John Clough: A Panel Discussion 9:00–10:30 am TextMusic Relations in Wolf 10:30–12:00 pm The Legacy of John Clough: New Research Directions, Part I 10:30am–12:00 Scriabin pm 12:00–1:45 pm Lunch 1:45–3:15 pm Set Theory 1:45–3:15 pm The Legacy of John Clough: New Research Directions, Part II 3:20–;4:50 pm Revisiting Established Harmonic and Formal Models 3:20–4:50 pm Harmony and Sound Play 4:55 pm Business Meeting & Reception Sunday, 10 April 8:30–9:00 am Registration 9:00–12:00 pm Rhythm and Meter in Brahms 9:00–12:00 pm Music After 1950 12:00–1:30 pm Lunch 1:30–3:00 pm Chromaticism Program Committee: Steven Laitz (Eastman School of Music), chair; Poundie Burstein (ex officio), Martha Hyde (University of Buffalo, SUNY) Eric McKee (Pennsylvania State University); Rebecca Jemian (Ithaca College), and Alexandra Vojcic (Juilliard). MTSNYS Home Page | Conference Information Saturday, 9:00–10:30 am The Legacy of John Clough: A Panel Discussion Chair: Norman Carey (Eastman School of Music) Jack Douthett (University at Buffalo, SUNY) Nora Engebretsen (Bowling Green State University) Jonathan Kochavi (Swarthmore, PA) Norman Carey (Eastman School of Music) John Clough was a pioneer in the field of scale theory. -

Appendix A: Mathematical Preliminaries
Appendix A: Mathematical Preliminaries Definition A1. Cartesian Product Let X and Y be two non-empty sets. The Cartesian product of X and Y, denoted X Â Y, is the set of all ordered pairs, a member of X followed by a member of Y. That is, ÈÉ X Â Y ¼ ðÞx, y x 2 X, y ∈ Y : Example A2. Let X ¼ {0, 1}, Y ¼ {x, y, z}. Then the Cartesian product X Â Y of X and Y is fgðÞ0, x ,0,ðÞy ,0,ðÞz ,1,ðÞx ,1,ðÞy ,1,ðÞz : Definition A3. Relation Let X and Y be two non-empty sets. A subset of X Â Y is a relation from X to Y; a subset of X Â X is a relation in X. Example A4. Let X ¼ {0, 1}, Y ¼ {x, y, z}. Then ÈÉÀ Á À Á À Á R ¼ ðÞ0, x , 1, x , 1, y , 1, z , R0 ¼ ∅, are relations from X to Y.(R0 is a relation because the empty set ∅ is a subset of every set.) Moreover, R00 ¼ fgðÞ0, 0 ,1,0ðÞ,1,1ðÞ is a relation in X. Definition A5. Function Let X and Y be two non-empty sets. E. Agmon, The Languages of Western Tonality, Computational Music Science, 265 DOI 10.1007/978-3-642-39587-1, © Springer-Verlag Berlin Heidelberg 2013 266 Appendix A: Mathematical Preliminaries A function f from X into Y is a subset of X Â Y (i.e., a relation from X to Y) with the following property. For every x ∈ X, there exists exactly one y ∈ Y, such that (x, y) ∈ f. -

MTO 22.4: Gotham, Pitch Properties of the Pedal Harp
Volume 22, Number 4, December 2016 Copyright © 2016 Society for Music Theory Pitch Properties of the Pedal Harp, with an Interactive Guide * Mark R. H. Gotham and Iain A. D. Gunn KEYWORDS: harp, pitch, pitch-class set theory, composition, analysis ABSTRACT: This article is aimed at two groups of readers. First, we present an interactive guide to pitch on the pedal harp for anyone wishing to teach or learn about harp pedaling and its associated pitch possibilities. We originally created this in response to a pedagogical need for such a resource in the teaching of composition and orchestration. Secondly, for composers and theorists seeking a more comprehensive understanding of what can be done on this unique instrument, we present a range of empirical-theoretical observations about the properties and prevalence of pitch structures on the pedal harp and the routes among them. This is particularly relevant to those interested in extended-tonal and atonal repertoires. A concluding section discusses prospective theoretical developments and analytical applications. 1 of 21 Received June 2016 I. Introduction [1.1] Most instruments have a relatively clear pitch universe: some have easy access to continuous pitch (unfretted string instruments, voices, the trombone), others are constrained by the 12-tone chromatic scale (most keyboards), and still others are limited to notes of the diatonic or pentatonic scales (many non-Western-orchestral members of the xylophone family). The pedal harp, by contrast, falls somewhere between those last two categories: it is organized around a diatonic configuration, but can reach all 12 notes of the chromatic scale, and most (but not all) pitch-class sets. -

Tipping the Scales . . . Functionally!
Tipping the Scales . Functionally! Gordon J. Pace and Kevin Vella Department of Computer Science, University of Malta Although one might be tempted to attribute the choice of notes in musical scales and chords to purely aesthetic considerations, a growing body of work seeks to establish correspondences with mathematical notions. Intriguingly, the most important musical scales turn out to be solutions to optimisation problems. In this abstract, we review mathematical characterisations which yield some of the most important musical scales. We also describe an implementation of these notions using the functional programming language Haskell, which has allowed us to conduct interactive experiments and to visualise various approaches from the literature. 1 Pitches, Pitch Classes and Scales The pitch of a musical note is quantified by its frequency. Pitches are partitioned into pitch classes with frequencies p × 2n; −∞ < n < 1 (doubling frequencies) under the octave equivalence relation. Pitches within the same pitch class, although having different frequencies, are perceived as being of the same quality [6]. The continuum of pitch classes can be discretised by choosing a finite subset of pitch classes as a palette. This is the chromatic pitch-class set1, the raw material at a composer’s disposition. Frequencies are typically chosen at (or near) equidistant points on a logarithmic scale, so that the brain perceives the distance between successive pairs of pitches as being the same. Though by no means universal, the use of twelve pitch classes is predominant in western music [3] and is widely represented in other cultures. Western music also exhibits a preference for scales containing seven note pitch-class sets chosen from the twelve-note chromatic set. -

Tuning, Tonality, and 22-Tone Temperament
Tuning, Tonality, and Twenty-Two-Tone Temperament Paul Erlich ([email protected]) (originally published in Xenharmonikôn 17, Spring 1998; revised 4/2/02) Introduction Western modal and tonal music generally conforms to what is known as the 5-limit; this is often explained (e. g., by Hindemith) by stating that the entire tuning system is an expression of integer frequency ratios using prime factors no higher than 5. A more correct description is that all 5-limit intervals, or ratios involving numbers no higher than 5, aside from factors of 2 arising from inversion and extension, are treated as consonances in this music, and thus need to be tuned with some degree of accuracy. The diatonic scale of seven notes per octave has been in use since ancient Greek times, when harmonic simultaneities (other than unisons and octaves) were not used and the only important property of the scale was its melodic structure. As melodic considerations continued to play a dominant role in Western musical history, scales other than the diatonic had very limited application, and harmonic usage was intimately entangled with diatonic scale structure. It is fortuitous that the diatonic scale allowed for many harmonies that displayed the psychoacoustic phenomenon of consonance, defined by small- integer frequency ratios, as well as many that did not. Medieval music conformed to the 3-limit, the only recognized consonances being the octave (2:1) and the perfect fifth (3:2) (plus, of course, their octave inversions and extensions, obtained by dividing or multiplying the ratios by powers of 2; this “octave equivalence” will be implicit from here on.1) The diatonic scales were therefore tuned as a chain of consecutive 3:2s (Pythagorean tuning). -

"T"-L--I Id Ie La Ib Lc Fig
BRIDGES Mathematical Connections in Art, Music, and Science Maximally Even Sets A discovery in mathematical music theory is found to apply in physics Richard Krantz Jack Douthett John Clough Department of Physics Department of Arts and Sciences Department of Music Metropolitan State College of TVI Community College SUNY Buffalo Denver Albuquerque, NM 87106 Buffalo, NY 14209 Denver, CO 80217 email: [email protected] ~mai1: ~mai1: [email protected] [email protected] 1. Introduction We wish to seat eight dinner guests--four women and four men--about a round table so that the guests of each gender are distributed as evenly as possible. The obvious solution is to seat men and women alterilately. Now suppose we have five women and three men. Ignoring rotations and reflections, there are essentially five ways to seat them (Fig. 1). Which of these is the most (maximally) even distribution? (We will see later that the optimum distribution for one gender guarantees the same for the other.) On the basis of the informal "most even" criterion, the best choice seems to be Fig. Ie (which happens to be the only arrangement that avoids seating three or more women together). This is a relatively simple case, but "dinner table" cases with larger numbers also have a unique best solution. How can we formalize our intuition about evenness for all such cases? JI ...•....f!! 11·· ...,.···· :f·····1f····~\ / ~~ 11 (1/ i ,~ ~ ,.: ·~·· .... ~ .. ···i ~·····~·····t "t"-l--i Id Ie la Ib lc Fig. 1: Seating A"angements 2. Defining Maximal Evenness There are many ways to defme maximal evenness consistent with the dinner guest problem above. -

Chord Proximity, Parsimony, and Analysis with Filtered Point-Symmetry
Chord Proximity, Parsimony, and Analysis with Filtered Point-Symmetry Richard Plotkin NOTE: The examples for the (text-only) PDF version of this item are available online at: hp://www.mtosmt.org/issues/mto.19.25.2/mto.19.25.2.plotkin.php KEYWORDS: maximal evenness, voice-leading parsimony, neo-Riemannian theory, transformational theory ABSTRACT: Filtered Point-Symmetry (FiPS) is a tool for modeling relationships between iterated maximally even sets. Common musical relationships can be studied by using FiPS to model chords contained within a specific scalar context (such as C major or the 01-octatonic collection), and by capturing those relationships in a FiPS configuration space. In this work, many FiPS configuration spaces are presented; some are isomorphic to commonly referenced voice-leading spaces like the neo-Riemannian Tonne, and others show tonal networks that have not previously been explored. A displacement operation is introduced to codify the traversal of a configuration space, and a short music analysis is provided to demonstrate some benefits of the approach. DOI: 10.30535/mto.25.2.3 Received April 2018 Volume 25, Number 2, July 2019 Copyright © 2019 Society for Music Theory [1.1] Scale theory and transformational theory have converged in some interesting and exciting ways in music theory, notably in the presentation of neo-Riemannian theory and other transformational theories involving frugal pitch-class changes (where “frugal” here is meant to include, but not exclusively refer to, parsimonious voice leading). An important visualization of this convergence is the neo-Riemannian Tonne, which ushered in a wave of thought about how music moves in time through a constructed (or generated) space. -
A Generalisation of Diatonicism and the Discrete Fourier Transform As a Mean for Classifying and Characterising Musical Scales
A Generalisation of Diatonicism and the Discrete Fourier Transform as a Mean for Classifying and Characterising Musical Scales Julien Junod1, Pierre Aud´etat2, Carlos Agon1, and Moreno Andreatta1 1 Equipe des Repr´esentations Musicales, IRCAM, Paris, France 2 Jazz Department, University of Applied Sciences Western Switzerland Abstract. Two approaches for characterising scales are presented and compared in this paper. The first one was proposed three years ago by the musician and composer Pierre Aud´etat, who developed a numerical and graphical representation of the 66 heptatonic scales and their 462 modes, a new cartography called the Diatonic Bell. It allows sorting and classifying the scales according to their similarity to the diatonic scale. The second approach uses the Discrete Fourier Transform (DFT) to investigate the geometry of scales in the chromatic circle. The study of its coefficients brings to light some scales, not necessarily the diatonic one, showing remarkable configurations. However, it does not lead to an evident classification, or linear ordering of scales. 1 Introduction Over centuries, western musicians have extensively used half a dozen of hepta- tonic scales, but combinatorics teach us that they represent only a tenth of the totally available musical material. Many catalogues exist, but they often reduce to numerical tables, that may not be easy to handle for composers. The musician and composer Pierre Aud´etat [2] developed a numerical and graphical representation of all 66 heptatonic scales and their 462 associated modes. Such a cartography, called the Diatonic Bell, opens a field of experiment equally relevant for composition and analysis, and presents interesting develop- ments for teaching. -

A Unified Theory of Chord Quality in Equal Temperaments by Ian Quinn Submitted in Partial Fulfillment of the Requirements for Th
A Unified Theory of Chord Quality in Equal Temperaments by Ian Quinn Submitted in partial fulfillment of the requirements for the degree Doctor of Philosophy Supervised by Professor Robert Morris Department of Music Theory Eastman School of Music University of Rochester Rochester, New York 2004 ii To the memory of David Lewin iii curriculum vitæ Ian Quinn was born at Warner-Robins AFB in Georgia on 19 March 1972. He was awarded the B.A. in music from Columbia University in 1993. After studies in the Ph.D. Program in Music at the CUNY Graduate Center under a Robert E. Gilleece Fellowship, he came to Eastman in 1996 with a Robert and Mary Sproull Fellowship from the University of Rochester, and was named Chief Master’s Marshal in 1999, having earned the M.A. in October 1998. In that same year he won the Edward Peck Curtis Award for Excellence in Teaching by a Graduate Student. He has held teaching assistantships at Eastman and instructorships both at Eastman and at the University of Rochester’s College Music Department. He left residence at the University to take the first postdoctoral fellowship in music theory at the University of Chicago (2002–03) and, subsequently, faculty appointments at the University of Oregon (2003–04) and Yale University (from 2004). He has performed on Nonesuch and Cantaloupe Records with Ossia, an ensemble he cofounded in 1997 that continues to play an active role in the new-music scene at Eastman. His research as a graduate student has been presented at many conferences and published in Music Theory Spectrum, Music Perception, and Perspectives of New Music. -

THE GEOMETRY of MUSICAL CHORDS Dmitri Tymoczko, Princeton University
THE GEOMETRY OF MUSICAL CHORDS Dmitri Tymoczko, Princeton University Musical chords have a non-Euclidean geometry that has been exploited by Western composers in many different styles. A musical chord can be represented as a point in a geometrical space called an orbifold. Line segments represent mappings from the notes of one chord to those of another. Composers in a wide range of styles have exploited the non-Euclidean geometry of these spaces, typically by utilizing short line segments between structurally similar chords. Such line segments exist only when chords are nearly symmetrical under translation, reflection, or permutation. Paradigmatically consonant and dissonant chords possess different near-symmetries, and suggest different musical uses. Western music lies at the intersection of two seemingly independent disciplines: harmony and counterpoint. Harmony delimits the acceptable chords (simultaneously occurring notes) and chord sequences. Counterpoint (or voice leading) is the technique of connecting the individual notes in a series of chords so as to form simultaneous melodies. Chords are usually connected so that these lines (or voices) move independently (not all in the same direction by the same amount), efficiently (by short distances), and without voice crossings (along non-intersecting paths) (Fig. 1A-C). These features facilitate musical performance, engage explicit aesthetic norms (1-2), and enable listeners to distinguish multiple simultaneous melodies (3). How is it that Western music can satisfy harmonic and contrapuntal constraints at once? What determines whether two chords can be connected by efficient voice leading? Musicians have been investigating these questions for almost three centuries. The circle of fifths (Fig. S1), first published in 1728 (4), depicts efficient voice leadings among the twelve major scales.