The Ecological Consequences and Adaptive Function
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Analgesic Effects and Pharmacologic Mechanisms of the Gelsemium
www.nature.com/scientificreports OPEN Analgesic efects and pharmacologic mechanisms of the Gelsemium alkaloid koumine on a Received: 8 June 2017 Accepted: 13 October 2017 rat model of postoperative pain Published: xx xx xxxx Bo-Jun Xiong1, Ying Xu1,2, Gui-Lin Jin1,2, Ming Liu1, Jian Yang1,2 & Chang-Xi Yu1,2 Postoperative pain (POP) of various durations is a common complication of surgical procedures. POP is caused by nerve damage and infammatory responses that are difcult to treat. The neuroinfammation- glia-steroid network is known to be important in POP. It has been reported that the Gelsemium alkaloid koumine possesses analgesic, anti-infammatory and neurosteroid modulating activities. This study was undertaken to test the analgesic efects of koumine against POP and explore the underlying pharmacologic mechanisms. Our results showed that microglia and astroglia were activated in the spinal dorsal horn post-incision, along with an increase of proinfammatory cytokines (interleukin 1β, interleukin 6, and tumor necrosis factor α). Both subcutaneous and intrathecal (i.t.) koumine treatment after incision signifcantly prevented mechanical allodynia and thermal hyperalgesia, inhibited microglial and astroglial activation, and suppressed expression of proinfammatory cytokines. Moreover, the analgesic efects of koumine were antagonized by i.t. administration of translocator protein (18 kDa) (TSPO) antagonist PK11195 and GABAA receptor antagonist bicuculline. Together, koumine prevented mechanical allodynia and thermal hyperalgesia caused by POP. The pharmacologic mechanism of koumine-mediated analgesia might involve inhibition of spinal neuroinfammation and activation of TSPO. These data suggested that koumine might be a potential pharmacotherapy for the management of POP. Postoperative pain (POP) of varying duration is extremely common afer surgery. -

Anthidium Manicatum, an Invasive Bee, Excludes a Native Bumble Bee, Bombus Impatiens, from floral Resources
Biol Invasions https://doi.org/10.1007/s10530-018-1889-7 (0123456789().,-volV)(0123456789().,-volV) ORIGINAL PAPER Anthidium manicatum, an invasive bee, excludes a native bumble bee, Bombus impatiens, from floral resources Kelsey K. Graham . Katherine Eaton . Isabel Obrien . Philip T. Starks Received: 15 April 2018 / Accepted: 21 November 2018 Ó Springer Nature Switzerland AG 2018 Abstract Anthidium manicatum is an invasive pol- response to A. manicatum presence. We found that B. linator reaching widespread distribution in North impatiens avoided foraging near A. manicatum in both America. Male A. manicatum aggressively defend years; but despite this resource exclusion, we found no floral territories, attacking heterospecific pollinators. evidence of fitness consequences for B. impatiens. Female A. manicatum are generalists, visiting many of These results suggest A. manicatum pose as significant the same plants as native pollinators. Because of A. resource competitors, but that B. impatiens are likely manicatum’s rapid range expansion, the territorial able to compensate for this resource loss by finding behavior of males, and the potential for female A. available resources elsewhere. manicatum to be significant resource competitors, invasive A. manicatum have been prioritized as a Keywords Exotic species Á Resource competition Á species of interest for impact assessment. But despite Interspecific competition Á Foraging behavior Á concerns, there have been no empirical studies inves- Pollination tigating the impact of A. manicatum on North Amer- ican pollinators. Therefore, across a two-year study, we monitored foraging behavior and fitness of the common eastern bumble bee (Bombus impatiens) in Introduction With increasing movement of goods and people Electronic supplementary material The online version of around the world, introduction of exotic species is this article (https://doi.org/10.1007/s10530-018-1889-7) con- increasing at an unprecedented rate (Ricciardi et al. -

Simvastatin Attenuates Renal Ischemia/Reperfusion Injury from Oxidative Stress Via Targeting Nrf2/HO‑1 Pathway
4460 EXPERIMENTAL AND THERAPEUTIC MEDICINE 14: 4460-4466, 2017 Simvastatin attenuates renal ischemia/reperfusion injury from oxidative stress via targeting Nrf2/HO‑1 pathway YU ZHANG1, SHU RONG2, YI FENG1, LIQUN ZHAO1, JIANG HONG1, RUILAN WANG1 and WEIJIE YUAN2 Departments of 1Emergency Intensive Medicine and 2Nephrology, Shanghai General Hospital of Nanjing Medical University, Shanghai 200082, P.R. China Received August 31, 2016; Accepted June 15, 2017 DOI: 10.3892/etm.2017.5023 Abstract. Ischemia-reperfusion (I/R) injury of the kidneys pathway to ultimately protect the kidneys from I/R-associated is commonly encountered in the clinic. The present study oxidative damage. assessed the efficacy of simvastatin in preventing I/R‑induced renal injury in a rat model and investigated the corresponding Introduction molecular mechanisms. Rats were divided into 3 groups, including a sham, I/R and I/R + simvastatin group. The Ischemia/reperfusion (I/R) injury of the kidneys represents a results revealed that in the I/R group, the levels of blood urea challenge in the clinic and is commonly encountered in renal nitrogen, serum creatinine and lactate dehydrogenase were transplantation, hemorrhagic shock, partial nephrectomy and significantly higher than those in the sham group, which accidental or iatrogenic trauma, brining about serious injury was significantly inhibited by simvastatin pre‑treatment. to multiple organs, including renal tubules, brain and heart (1). I/R significantly decreased superoxide dismutase activity Therefore, it is urgent to find effective therapies and elucidate compared with that in the sham group, which was largely molecular mechanisms by which renal I/R injury may be rescued by simvastatin. -
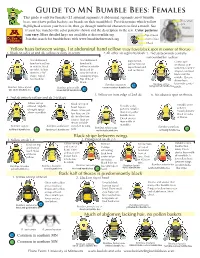
Guide to MN Bumble Bees: Females
Guide to MN Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble Three small bees, most have pollen baskets, no beards on their mandibles). First determine which yellow eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns ® can vary. More detailed keys are available at discoverlife.org. Top of head Bee Front of face Squad Join the search for bumble bees with www.bumbleebeewatch.org Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee C patch. two-spotted bumble bee C brown-belted bumble bee C 5. Yellow on front edge of 2nd ab 6. No obvious spot on thorax. 4. 2nd ab entirely yellow and ab 3-6 black Yellow on top Black on top of Variable color of head. -

High Throughput Determination of Multiple Toxic Alkaloids in Food By
Application Note Food Testing & Agriculture High-Throughput Determination of Multiple Toxic Alkaloids in Food by UHPLC/MS/MS Authors Abstract Guoyin Lai, Lijian Wu, Zhongda Lin, Liyi Lin, Dunming Xu, This Application Note presents a simple sample preparation procedure followed Zhigang Zhang with an Agilent 1290 Infinity II LC combined with an Agilent 6470A triple quadrupole Technigue Center, LC/MS for simultaneous, high-throughput determination of multiple alkaloids in food Xiamen Customs, matrices. The matrix-matched calibration shows very good linear relationships for Xiamen, China the 18 alkaloids in the concentration range of 0.5 to 50 µg/L in the diluted, extracted matrices of bread, milk, wine, and rice powder. This corresponds to 5.0 to 500 µg/kg Meiling Lu in the matrices when considering the dilution factor of 10. The linear regression Agilent Technologies (China) coefficients for all the analytes are higher than 0.99. The signal-to-noise ratios Co., Ltd. (S/Ns) for all analytes are higher than 10 at the lowest calibration level of 0.5 µg/L, corresponding to 5.0 µg/kg in the samples. The recoveries for the analytes in four different matrices at 5, 50, and 250 µg/kg levels ranged from 90 to 110%, and the corresponding relative standard deviations were within 2.3 to 7.9%. The results demonstrated that the developed method has the advantages of high sensitivity, accuracy, and precision. The simple sample preparation combined with rapid LC/MS/MS detection allows high-throughput screening of alkaloid residues in food matrices. Introduction Experimental (pH 3). The tube was vortexed thoroughly for two minutes, then centrifuged Alkaloids are naturally occurring alkaline Standards and sample at 7,500 rpm for five minutes. -

Bumble Bee Abundance in New York City Community Gardens: Implications for Urban Agriculture
Matteson and Langellotto: URBAN BUMBLE BEE ABUNDANCE Cities and the Environment 2009 Volume 2, Issue 1 Article 5 Bumble Bee Abundance in New York City Community Gardens: Implications for Urban Agriculture Kevin C. Matteson and Gail A. Langellotto Abstract A variety of crops are grown in New York City community gardens. Although the production of many crops benefits from pollination by bees, little is known about bee abundance in urban community gardens or which crops are specifically dependent on bee pollination. In 2005, we compiled a list of crop plants grown within 19 community gardens in New York City and classified these plants according to their dependence on bee pollination. In addition, using mark-recapture methods, we estimated the abundance of a potentially important pollinator within New York City urban gardens, the common eastern bumble bee (Bombus impatiens). This species is currently recognized as a valuable commercial pollinator of greenhouse crops. However, wild populations of B. impatiens are abundant throughout its range, including in New York City community gardens, where it is the most abundant native bee species present and where it has been observed visiting a variety of crop flowers. We conservatively counted 25 species of crop plants in 19 surveyed gardens. The literature suggests that 92% of these crops are dependent, to some degree, on bee pollination in order to set fruit or seed. Bombus impatiens workers were observed visiting flowers of 78% of these pollination-dependent crops. Estimates of the number of B. impatiens workers visiting individual gardens during the study period ranged from 3 to 15 bees per 100 m2 of total garden area and 6 to 29 bees per 100 m2 of garden floral area. -

Conserving Missouri's Wild and Managed Pollinators
Conserving Missouri’s Wild and Managed Pollinators At the heart of the pollination issue lies our bounty of foods such as peaches, Go to the bee, thou poet: strawberries, squash and apples. These and other foods requiring pollination have consider her ways and be wise. been staples in the human diet for centuries, and their pollinators have been highly — George Bernard Shaw revered since ancient times. From Egyptian hieroglyphics and Native American cave paintings to Greek mythology and English poetry, bees and butterflies have been a source of fascination and awe for millennia (Figure 1). Yet, over the past century, pollinator numbers have suffered declines. During this time, global development, a booming human population, and industrial agriculture brought about drastic landscape changes. These changes have resulted in greater losses of forage and nesting resources for pollinators than ever before seen. The ecosystem service of pollination, once taken for granted, is now potentially threatened as many pollinator species face declines in Missouri, the United States and many regions around the world. Pollinators are critically important for natural ecosystems and crop production. Through pollination, they perform roles essential to human welfare. Heightened public awareness of their services and of their declines over the past two decades has prompted action, but much remains to be done. This publication introduces issues regarding the conservation of pollinators in Missouri. It explores why pollinators are crucial, what major threats confront them, what conservation steps are being taken, and how you can help. It highlights bees over other pollinators as bees are the most important for both agricultural and natural pollination in Missouri. -

Comparative Analysis of Gelsemine and Gelsemium Sempervirens Activity on Neurosteroid Allopregnanolone Formation in the Spinal Cord and Limbic System
Hindawi Publishing Corporation Evidence-Based Complementary and Alternative Medicine Volume 2011, Article ID 407617, 10 pages doi:10.1093/ecam/nep083 Original Article Comparative Analysis of Gelsemine and Gelsemium sempervirens Activity on Neurosteroid Allopregnanolone Formation in the Spinal Cord and Limbic System Christine Venard,1 Naoual Boujedaini,2 Ayikoe Guy Mensah-Nyagan,1 and Christine Patte-Mensah1 1 Equipe “St´ero¨ıdes, Neuromodulateurs et Neuropathologies”, EA-4438 Universit´edeStrasbourg,Bˆatiment 3 de la Facult´edeM´edecine, F-67000 Strasbourg, France 2 Laboratoires BOIRON, Sainte-Foy-l`es-Lyon, France Correspondence should be addressed to Ayikoe Guy Mensah-Nyagan, [email protected] Received 10 February 2009; Accepted 15 June 2009 Copyright © 2011 Christine Venard et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Centesimal dilutions (5, 9 and 15 cH) of Gelsemium sempervirens are claimed to be capable of exerting anxiolytic and analgesic effects. However, basic results supporting this assertion are rare, and the mechanism of action of G. sempervirens is completely unknown. To clarify the point, we performed a comparative analysis of the effects of dilutions 5, 9 and 15 cH of G. sempervirens or gelsemine (the major active principle of G. sempervirens) on allopregnanolone (3α,5α-THP)productionintheratlimbicsystem (hippocampus and amygdala or H-A) and spinal cord (SC). Indeed, H-A and SC are two pivotal structures controlling, respectively, anxiety and pain that are also modulated by the neurosteroid 3α,5α-THP. At the dilution 5 cH, both G. -

Bombus Impatiens, Common Eastern Bumblebee
Bombus impatiens, Common Eastern Bumble Bee (Hymenoptera: Apidae) Christopher J. Fellows, Forest Huval, T.E. Reagan and Chris Carlton Description The common eastern bumble bee, Bombus impatiens, is an important native pollinator found in the eastern United States and southern Canada. Adult bees are covered with short, even hairs across their bodies. They are mostly black with a yellow thorax (middle section) and an additional yellow stripe at the base of the abdomen. As with other social insects, the different forms, or castes, of B. impatiens vary in size. Worker bumble bees measure from 1/3 to 2/3 of an inch in length (8.5-16 mm), while drones measure ½ to ¾ of an inch (12 to 18 mm). Queen bumble bees are the largest members of the three castes, measuring between 1/3 of an inch to 1 inch (17 and 23 mm) in length. Larvae are rarely seen and are enclosed with the nest brood cells for the duration of their A common eastern bumble bee resting on a flower. Note development. They are pale, legless grubs around 1 inch the yellow patch of hairs on the forward portion of the thorax (David Cappaert, Bugwood.org). (25 mm) in length. At least six additional species of bumble bees have been construction. Within the hollow fiber ball, the queen documented in Louisiana, with another two species possibly deposits a lump of nectar-moistened pollen. The queens present but not confirmed. Identifications are mainly based also construct honey pots near the nest entrance using on the patterns of coloration of the body hairs, but these the secretions of her wax glands. -

Honey Ingredients As Used in Cosmetics
Safety Assessment of Honey ingredients as Used in Cosmetics Status: Scientific Literature Review for Public Comment Release Date: September 3, 2019 Panel Meeting Date: December 9-10, 2019 All interested persons are provided 60 days from the above release date (November 2, 2019) to comment on this safety assessment and to identify additional published data that should be included or provide unpublished data which can be made public and included. Information may be submitted without identifying the source or the trade name of the cosmetic product containing the ingredient. All unpublished data submitted to CIR will be discussed in open meetings, will be available at the CIR office for review by any interested party and may be cited in a peer-reviewed scientific journal. Please submit data, comments, or requests to the CIR Executive Director, Dr. Bart Heldreth. The 2019 Cosmetic Ingredient Review Expert Panel members are: Chair, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; James G. Marks, Jr., M.D., Ronald C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. The CIR Executive Director is Bart Heldreth, Ph.D. This safety assessment was prepared by Priya Cherian, Scientific Analyst/Writer. © Cosmetic Ingredient Review 1620 L Street, NW, Suite 1200 ♢ Washington, DC 20036-4702 ♢ ph 202.331.0651 ♢ fax 202.331.0088 ♢ [email protected] INTRODUCTION This scientific literature review is the initial step in preparing a safety assessment of the following 7 honey ingredients as used in cosmetic formulations: Honey Honey Powder Hydrogenated Honey Hydrolyzed Honey Protein Honey Cocoates Honey Extract Hydrolyzed Honey According to the web-based International Cosmetic Ingredient Dictionary and Handbook (wINCI; Dictionary), all of these ingredients function as skin-conditioning agents.1 Other functions include, but are not limited to, use as a flavoring agent, anti-acne agent, abrasive, binder, depilating agent, exfoliant, hair-conditioning agent, and nail-conditioning agent (Table 1). -

Creating Economically and Ecologically Sustainable Pollinator Habitat District 2 Demonstration Research Project Summary Updated for Site Visit in April 2019
Creating Economically and Ecologically Sustainable Pollinator Habitat District 2 Demonstration Research Project Summary Updated for Site Visit in April 2019 The PIs are most appreciative for identification assistance provided by: Arian Farid and Alan R. Franck, Director and former Director, resp., University of South Florida Herbarium, Tampa, FL; Edwin Bridges, Botanical and Ecological Consultant; Floyd Griffith, Botanist; and Eugene Wofford, Director, University of Tennessee Herbarium, Knoxville, TN Investigators Rick Johnstone and Robin Haggie (IVM Partners, 501-C-3 non-profit; http://www.ivmpartners.org/); Larry Porter and John Nettles (ret.), District 2 Wildflower Coordinator; Jeff Norcini, FDOT State Wildflower Specialist Cooperator Rick Owen (Imperiled Butterflies of Florida Work Group – North) Objective Evaluate a cost-effective strategy for creating habitat for pollinators/beneficial insects in the ROW beyond the back-slope. Rationale • Will aid FDOT in developing a strategy to create pollinator habitat per the federal BEE Act and FDOT’s Wildflower Program • Will demonstrate that FDOT can simultaneously • Create sustainable pollinator habitat in an economical and ecological manner • Reduce mowing costs • Part of national effort coordinated by IVM Partners, who has • Established or will establish similar projects on roadside or utility ROWS in Alabama, Arkansas, Maryland, New Mexico, Oklahoma, Idaho, Montana, Virginia, West Virginia, and Tennessee; studies previously conducted in Arizona, Delaware, Michigan, and New Jersey • Developed partnerships with US Fish & Wildlife Service, Army Corps of Engineers, US Geological Survey, New Jersey Institute of Technology, Rutgers University, Chesapeake Bay Foundation, Chesapeake Wildlife Heritage, The Navajo Nation, The Wildlife Habitat Council, The Pollinator Partnership, Progressive Solutions, Bayer Crop Sciences, Universities of Maryland, Ohio, West Virginia, and the EPA. -

Bumble Bees Are Essential
Prepared by the Bombus Task Force of the North American Pollinator Protection Campaign (NAPPC) Photo Sheila Colla Rusty-patched bumble bee, Bombus affi nis Declining North American Bumble Bees Photo David Inouye Western bumble bee, Bombus occidentalis Bumble Bees Helping Photo James Strange are Pollinators Essential Thrive Franklin bumble bee, Bombus franklini Bumble Bee Facts Photo Leif Richardson Globally, there are about 250 described species Some bumble bee are known to rob fl owers of bumble bees. They are found primarily in the of their nectar. Nectar robbing occurs when temperate zones of North and South America, a bee extracts nectar from a fl ower without and Eurasia. coming into contact with its reproductive Yellow-banded bumble bee, Bumble bees are documented to pollinate parts (i.e. anthers and/or stigma), usually Bombus terricola many important food crops. They are also more by biting a hole at the base of the fl ower. Photo Ron Hemberger effective than honey bees at pollinating crops Bumble bees are effective buzz pollinators grown in greenhouses. of several economically important plants in When most insects are inactive due to cold the family Solanaceae such as tomato, bell temperatures bumble bees are able to fl y by pepper and eggplant. In buzz pollination warming their fl ight muscles by shivering, bees extract pollen from a fl ower by American bumble bee, enabling them to raise their body temperature vibrating against the fl ower’s anthers, Bombus pensylvanicus as necessary for fl ight. making an audible buzzing noise. Instead of starting their own colonies, some Currently, the Common Eastern bumble bee bumble bee species have evolved to take over (Bombus impatiens) is the only species being another species’ colony to rear their young.