Xenopus Laevisafrican Clawed Frog
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Disease and Clinical Signs Poster
African Clawed Frog Xenopus laevis Diseases and Clinical Signs in Photographs Therese Jones-Green University Biomedical Services Abstract Reported clinical signs associated with ill health and Clinical signs found between 2016 to 2018 Many diseases of Xenopus laevis frogs have been described in the • Opened in 2005, the biofacility can hold up to 900 female and 240 disease Clinical signs found between 2016 to 2018 male frogs in a Marine Biotech (now serviced by MBK Installations Ltd.) • 80 recirculating aquatic system. • Buoyancy problems 70 bridge this gap using photographs taken of frogs with clinical signs. The system monitors parameters such as: temperature, pH, • • Changes in activity e.g. lethargy or easily startled. My aims are: conductivity, and Total Gas Pressure. 60 • Unusual amount of skin shedding. 2018 • To share my experiences, via images, to help improve the welfare and • Frogs are maintained on mains fed water with a 20% water exchange. 50 ases • Weight loss, or failure to feed correctly. c husbandry of this species. • 2017 • Coelomic (body cavity) distention. 40 • To stimulate a dialogue which results in further sharing of ideas and 2016 Whole body bloat. 30 information. • Frogs are held under a 12 hour light/dark cycle. • Number of • Each tank has a PVC enrichment tube (30cm x 10cm), with smoothed • Fungal strands/tufts. 20 Introduction cut edges. • Sores or tremors at extremities (forearms and toes). 10 Between January 2016 and October 2018, cases of ill health and disease • Frogs are fed three times a week with a commercial trout pellet. • Redness or red spots. 0 Oedema Red leg Red leg (bad Red leg Gas bubble Sores on forearm Opaque cornea Skeletal Growth (cartilage Scar Cabletie Missing claws Eggbound in a colony of Xenopus laevis frogs housed by the University Biological hormone) (nematodes) disease deformity under skin) • The focus of the research undertaken in the biofacility is embryonic and • Excessive slime/mucous production or not enough (dry dull skin). -

Table S1. Nakharuthai and Srisapoome (2020)
Primer name Nucleotide sequence (5’→3’) Purpose CXC-1F New TGAACCCTGAGCTGAAGGCCGTGA Real-time PCR CXC-1R New TGAAGGTCTGATGAGTTTGTCGTC Real-time PCR CXC-1R CCTTCAGCTCAGGGTTCAAGC Genomic PCR CXC-2F New GCTTGAACCCCGAGCTGAAAAACG Real-time PCR CXC-2R New GTTCAGAGGTCGTATGAGGTGCTT Real-time PCR CXC-2F CAAGCAGGACAACAGTGTCTGTGT 3’RACE CXC-2AR GTTGCATGATTTGGATGCTGGGTAG 5’RACE CXC-1FSB AACATATGTCTCCCAGGCCCAACTCAAAC Southern blot CXC-1RSB CTCGAGTTATTTTGCACTGATGTGCAA Southern blot CXC1Exon1F CAAAGTGTTTCTGCTCCTGG Genomic PCR On-CXC1FOverEx CATATGCAACTCAAACAAGCAGGACAACAGT Overexpression On-CXC1ROverEx CTCGAGTTTTGCACTGATGTGCAATTTCAA Overexpression On-CXC2FOverEx CATATGCAACTCAAACAAGCAGGACAACAGT Overexpression On-CXC2ROverEx CTCGAGCATGGCAGCTGTGGAGGGTTCCAC Overexpression β-actinrealtimeF ACAGGATGCAGAAGGAGATCACAG Real-time PCR β-actinrealtimeR GTACTCCTGCTTGCTGATCCACAT Real-time PCR M13F AAAACGACGGCCAG Nucleotide sequencing M13R AACAGCTATGACCATG Nucleotide sequencing UPM-long (0.4 µM) CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT RACE PCR UPM-short (2 µM) CTAATAC GACTCACTATA GGGC RACE PCR Table S1. Nakharuthai and Srisapoome (2020) On-CXC1 Nucleotide (%) Amino acid (%) On-CXC2 Nucleotide (%) Amino acid (%) Versus identity identity similarity Versus identity identity Similarity Teleost fish 1. Rock bream, Oplegnathus fasciatus (AB703273) 64.5 49.1 68.1 O. fasciatus 70.7 57.7 75.4 2. Mandarin fish, Siniperca chuatsi (AAY79282) 63.2 48.1 68.9 S. chuatsi 70.5 54.0 78.8 3. Atlantic halibut, Hippoglossus hippoglossus (ACY54778) 52.0 39.3 51.1 H. hippoglossus 64.5 46.3 63.9 4. Common carp IL-8, Cyprinus carpio (ABE47600) 44.9 19.1 34.1 C. carpio 49.4 21.9 42.6 5. Rainbow trout IL-8, Oncorhynchus mykiss (CAC33585) 44.0 21.3 36.3 O. mykiss 47.3 23.7 44.4 6. Japanese flounder IL-8, Paralichthys olivaceus (AAL05442) 48.4 25.4 45.9 P. -

Xenopus for Dummies.Docx
Xenopus for DUMMIES 1 Xenopus for DUMMIES OUTLINE Introduction ........................................................................................................................... 2 1. Why is Xenopus interesting for iGEM? ........................................................................... 4 2. Requirements before planning to work on Xenopus......................................................... 5 3. Guidelines to define an iGEM project with Xenopus ........................................................ 5 4. The micro-injection procedure ........................................................................................ 7 a. Step 1: Fertilized eggs ........................................................................................ 7 b. Step 2: DNA injection .......................................................................................... 7 c. Place the injected embryos in incubator 21°C ..................................................... 8 d. The following days .............................................................................................. 8 e. Embryos injection: efficiency ............................................................................... 8 5. The biobrick plasmids for Xenopus ................................................................................. 9 6. Xenopus debugging tool ............................................................................................... 10 a. How? ................................................................................................................ -

Association of DNA with Nuclear Matrix in in Vitro Assembled Nuclei
Cell Research (1997), 7, 107-117 Association of DNA with nuclear matrix in in vitro as- sembled nuclei induced by rDNA from Tetrahymena shang- haiensis in Xenopus egg extracts CHEN YING, BO ZHANG, XIU FEN LI, ZHONG HE ZHAI Department of Cell Biology and Genetics, College of Life Sciences, Peking University, Beijing 100871 ABSTRACT The nuclei assembled from exogenous DNA or chro- matin in egg extracts resemble their in vivo counterparts in many aspects. However, the distribution pattern of DNA in these nuclei remains unknown. We introduced rDNA from the macronuclei of Tetrahymena into Xenopus cell- free extracts to examine the association of specific DNA sequences with nuclear matrix (NM) in the nuclei assem- bled in vitro. Our previous works showed the 5'NTS (non- transcription sequences) of the rDNA specifically bind to the NM system in the macronuclei. We show now the rDNA could induce chromatin assembly and nuclear for- mation in Xenopus cell-free system. When we extracted the NM system and compared the binding affinity of differ- ent regions of rDNA with the NM system, we found that the 5'NTS still hold their binding affinity with insoluble structure of the assembled nuclei in the extracts of Xeno- pus eggs. Key words: Nuclear assembly, nuclear matrix, Xeno- pus egg extracts, Tetrahymena rDNA. On the occasion of Professor Lu Ji SHI's (L. C. Sze), eightieth birthday, we present this paper and extend our sincere greetings to Professor SHI. As we mentioned in our paper, in early 1950's, it is Professor SHI who first studied the behavior of exogenous homologous desoxyribose nucleoprotein (chromatin) in amphibian eggs. -

Enhanced XAO: the Ontology of Xenopus Anatomy And
Segerdell et al. Journal of Biomedical Semantics 2013, 4:31 http://www.jbiomedsem.com/content/4/1/31 JOURNAL OF BIOMEDICAL SEMANTICS RESEARCH Open Access Enhanced XAO: the ontology of Xenopus anatomy and development underpins more accurate annotation of gene expression and queries on Xenbase Erik Segerdell1*, Virgilio G Ponferrada2, Christina James-Zorn2, Kevin A Burns2, Joshua D Fortriede2, Wasila M Dahdul3,4, Peter D Vize5 and Aaron M Zorn2 Abstract Background: The African clawed frogs Xenopus laevis and Xenopus tropicalis are prominent animal model organisms. Xenopus research contributes to the understanding of genetic, developmental and molecular mechanisms underlying human disease. The Xenopus Anatomy Ontology (XAO) reflects the anatomy and embryological development of Xenopus. The XAO provides consistent terminology that can be applied to anatomical feature descriptions along with a set of relationships that indicate how each anatomical entity is related to others in the embryo, tadpole, or adult frog. The XAO is integral to the functionality of Xenbase (http://www. xenbase.org), the Xenopus model organism database. Results: We significantly expanded the XAO in the last five years by adding 612 anatomical terms, 2934 relationships between them, 640 synonyms, and 547 ontology cross-references. Each term now has a definition, so database users and curators can be certain they are selecting the correct term when specifying an anatomical entity. With developmental timing information now asserted for every anatomical term, the ontology provides internal checks that ensure high-quality gene expression and phenotype data annotation. The XAO, now with 1313 defined anatomical and developmental stage terms, has been integrated with Xenbase expression and anatomy term searches and it enables links between various data types including images, clones, and publications. -

Stages of Embryonic Development of the Zebrafish
DEVELOPMENTAL DYNAMICS 2032553’10 (1995) Stages of Embryonic Development of the Zebrafish CHARLES B. KIMMEL, WILLIAM W. BALLARD, SETH R. KIMMEL, BONNIE ULLMANN, AND THOMAS F. SCHILLING Institute of Neuroscience, University of Oregon, Eugene, Oregon 97403-1254 (C.B.K., S.R.K., B.U., T.F.S.); Department of Biology, Dartmouth College, Hanover, NH 03755 (W.W.B.) ABSTRACT We describe a series of stages for Segmentation Period (10-24 h) 274 development of the embryo of the zebrafish, Danio (Brachydanio) rerio. We define seven broad peri- Pharyngula Period (24-48 h) 285 ods of embryogenesis-the zygote, cleavage, blas- Hatching Period (48-72 h) 298 tula, gastrula, segmentation, pharyngula, and hatching periods. These divisions highlight the Early Larval Period 303 changing spectrum of major developmental pro- Acknowledgments 303 cesses that occur during the first 3 days after fer- tilization, and we review some of what is known Glossary 303 about morphogenesis and other significant events that occur during each of the periods. Stages sub- References 309 divide the periods. Stages are named, not num- INTRODUCTION bered as in most other series, providing for flexi- A staging series is a tool that provides accuracy in bility and continued evolution of the staging series developmental studies. This is because different em- as we learn more about development in this spe- bryos, even together within a single clutch, develop at cies. The stages, and their names, are based on slightly different rates. We have seen asynchrony ap- morphological features, generally readily identi- pearing in the development of zebrafish, Danio fied by examination of the live embryo with the (Brachydanio) rerio, embryos fertilized simultaneously dissecting stereomicroscope. -

Helical Insertion of Peptidoglycan Produces Chiral Ordering of the Bacterial Cell Wall
Helical insertion of peptidoglycan produces PNAS PLUS chiral ordering of the bacterial cell wall Siyuan Wanga,b, Leon Furchtgottc,d, Kerwyn Casey Huangd,1, and Joshua W. Shaevitza,e,1 aLewis-Sigler Institute for Integrative Genomics, Princeton University, Princeton, NJ 08544; bDepartment of Molecular Biology, Princeton University, Princeton, NJ 08544; cBiophysics Program, Harvard University, Cambridge, MA 02138; dDepartment of Bioengineering, Stanford University, Stanford, CA 94305; and eDepartment of Physics, Princeton University, Princeton, NJ 08854 AUTHOR SUMMARY Cells from all kingdoms of wall growth to demonstrate life face the task of that patterning of cell-wall constructing a specific, synthesis by left-handed mechanically robust three- MreB polymers leads to a dimensional (3D) cell shape right-handed chiral from molecular-scale organization of the glycan components. For many strands. This organization bacteria, maintaining a rigid, produces a left-handed rod-like shape facilitates a twisting of the cell body diverse range of behaviors during elongational growth. including swimming motility, We then confirm the detection of chemical existence of right-handed gradients, and nutrient access Fig. P1. Helical insertion of material into the bacterial cell wall (green) glycan organization in E. coli during elongational growth, guided by the protein MreB (yellow), leads and waste evacuation in by osmotically shocking biofilms. The static shape of to an emergent chiral order in the cell-wall network and twisting of the cell that can be visualized using surface-bound beads (red). surface-labeled cells and a bacterial cell is usually directly measuring the defined by the cell wall, a difference in stiffness macromolecular polymer network composed of glycan strands between the longitudinal and transverse directions. -

High Abundance of Invasive African Clawed Frog Xenopus Laevis in Chile: Challenges for Their Control and Updated Invasive Distribution
Management of Biological Invasions (2019) Volume 10, Issue 2: 377–388 CORRECTED PROOF Research Article High abundance of invasive African clawed frog Xenopus laevis in Chile: challenges for their control and updated invasive distribution Marta Mora1, Daniel J. Pons2,3, Alexandra Peñafiel-Ricaurte2, Mario Alvarado-Rybak2, Saulo Lebuy2 and Claudio Soto-Azat2,* 1Vida Nativa NGO, Santiago, Chile 2Centro de Investigación para la Sustentabilidad & Programa de Doctorado en Medicina de la Conservación, Facultad de Ciencias de la Vida, Universidad Andres Bello, Republica 440, Santiago, Chile 3Facultad de Ciencias Exactas, Departamento de Matemáticas, Universidad Andres Bello, Santiago, Republica 470, Santiago, Chile Author e-mails: [email protected] (CSA), [email protected] (MM), [email protected] (DJP), [email protected] (APR), [email protected] (MAR), [email protected] (SL) *Corresponding author Citation: Mora M, Pons DJ, Peñafiel- Ricaurte A, Alvarado-Rybak M, Lebuy S, Abstract Soto-Azat C (2019) High abundance of invasive African clawed frog Xenopus Invasive African clawed frog Xenopus laevis (Daudin, 1802) are considered a major laevis in Chile: challenges for their control threat to aquatic environments. Beginning in the early 1970s, invasive populations and updated invasive distribution. have now been established throughout much of central Chile. Between September Management of Biological Invasions 10(2): and December 2015, we studied a population of X. laevis from a small pond in 377–388, https://doi.org/10.3391/mbi.2019. 10.2.11 Viña del Mar, where we estimated the population size and evaluated the use of hand nets as a method of control. First, by means of a non-linear extrapolation Received: 26 October 2018 model using the data from a single capture session of 200 min, a population size of Accepted: 7 March 2019 1,182 post-metamorphic frogs (range: 1,168–1,195 [quadratic error]) and a density Published: 16 May 2019 of 13.7 frogs/m2 (range: 13.6–13.9) of surface water were estimated. -
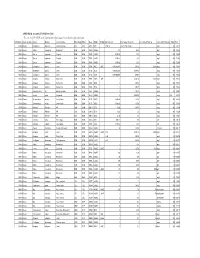
APPENDIX K: Accepted ECOTOX Data Table
APPENDIX K: Accepted ECOTOX Data Table The code list for ECOTOX can be found at: http://cfpub.epa.gov/ecotox/blackbox/help/codelist.pdf CAS Number Chemical Name Genus Species Common Name Effect Group Effect Meas Endpt1 Endpt2 Dur Preferred Conc Value1 Preferred Conc Value2 Preferred Conc Units Preferred % Purity Ref # 330541 Diuron Pimephales promelas Fathead minnow ACC ACC GACC BCF 1 TO 24 0.00315 TO 0.0304 mg/L 100 12612 330541 Diuron Rattus norvegicus Norway rat BCM BCM PHPH NOAEL 140 2500 ppm 100 90555 330541 Diuron Zostera capricorni Eelgrass BCM BCM FLRS LOAEL 8.33E-02 0.01 mg/L 100 72996 330541 Diuron Zostera capricorni Eelgrass BCM BCM FLRS LOAEL 8.33E-02 0.01 mg/L 100 72996 330541 Diuron Zostera capricorni Eelgrass BCM BCM FLRS LOAEL 8.33E-02 0.01 mg/L 100 72996 330541 Diuron Seriatopora hystrix Coral BCM BCM FLRS NOEC LOEC 0.416666667 0.0003 0.0003 mg/L 100 75334 330541 Diuron Seriatopora hystrix Coral BCM BCM FLRS EC25 0.416666667 0.00085 mg/L 100 75334 330541 Diuron Seriatopora hystrix Coral BCM BCM FLRS EC50 0.416666667 0.0023 mg/L 100 75334 330541 Diuron Acropora formosa Stony coral BCM BCM FLRS NOEC LOEC 1 0.00003 0.0003 mg/L 100 75334 330541 Diuron Acropora formosa Stony coral BCM BCM FLRS EC25 1 0.0012 mg/L 100 75334 330541 Diuron Acropora formosa Stony coral BCM BCM FLRS EC50 1 0.0027 mg/L 100 75334 330541 Diuron Synechococcus sp. Blue-green algae BCM BCM CHCT NOAEL 3 0.0033 mg/L 99 98904 330541 Diuron Lemna minor Duckweed BCM BCM GLTH LOAEL 2 0.02475 mg/L >99 64164 330541 Diuron Scenedesmus acutus Green Algae BCM BCM -

Xenopus Laevis
Guidance on the housing and care of the African clawed frog Xenopus laevis Barney T Reed Research Animals Department - RSPCA Guidelines for the housing and care of the African clawed frog (Xenopus laevis) May 2005 Guidance on the housing and care of the African clawed frog Xenopus laevis) Acknowledgements The author would like to sincerely thank the following people for their helpful and constructive comments during the preparation of this report: Mr. D. Anderson - Home Office, Animals Scientific Procedures Division Mr. M. Brown - MRC Laboratory of Molecular Biology, Cambridge Dr. G. Griffin - Canadian Council for Animal Care (CCAC) Dr. M. Guille - School of Biological Sciences, University of Portsmouth Dr. P. Hawkins - Research Animals Department, RSPCA Dr. R. Hubrecht - Universities Federation for Animal Welfare (UFAW) Dr. M. Jennings - Research Animals Department, RSPCA Dr. K. Mathers - MRC National Institute for Medical Research Dr. G. Sanders - School of Medicine, University of Washington Prof. R. Tinsley - School of Biological Sciences, University of Bristol Special thanks also to those organisations whose establishments I visited and whose scientific and animal care staff provided valuable input and advice. Note: The views expressed in this document are those of the author, and may not necessarily represent those of the persons named above or their affiliated organisations. About the author Barney Reed studied psychology and biology at the University of Exeter before obtaining a MSc in applied animal behaviour and animal welfare from the University of Edinburgh. He worked in the Animals Scientific Procedures Division of the Home Office before joining the research animals department of the RSPCA as a scientific officer. -

Xenopus in the Amphibian Ancestral Organization of the MHC Revealed
Ancestral Organization of the MHC Revealed in the Amphibian Xenopus Yuko Ohta, Wilfried Goetz, M. Zulfiquer Hossain, Masaru Nonaka and Martin F. Flajnik This information is current as of September 26, 2021. J Immunol 2006; 176:3674-3685; ; doi: 10.4049/jimmunol.176.6.3674 http://www.jimmunol.org/content/176/6/3674 Downloaded from References This article cites 70 articles, 21 of which you can access for free at: http://www.jimmunol.org/content/176/6/3674.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on September 26, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2006 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Ancestral Organization of the MHC Revealed in the Amphibian Xenopus1 Yuko Ohta,2* Wilfried Goetz,* M. Zulfiquer Hossain,* Masaru Nonaka,† and Martin F. Flajnik* With the advent of the Xenopus tropicalis genome project, we analyzed scaffolds containing MHC genes. On eight scaffolds encompassing 3.65 Mbp, 122 MHC genes were found of which 110 genes were annotated. -

Danio Rerio) Notum1b, Notum1a, and Notum2; Fruit Fly (Drosophila Melanogaster) Notum; and African Clawed Frog (Xenopus Laevis) Notum
Figure S1: Protein alignments of NOTUM homologs. Amino acid alignments of: human (Homo sapien) NOTUM; zebrafish (Danio rerio) notum1b, notum1a, and notum2; fruit fly (Drosophila melanogaster) notum; and African clawed frog (Xenopus laevis) notum. Note the high conservation around the active site motif G-X-S-X-G (blue box) and the Ser, Asp, His catalytic triad (red boxes) characteristic of the α/β hydrolase protein family. Table S1: Oligonucleotides utilized for qRT-PCR, clone sequencing, ISH, sgRNA synthesis, and genotyping. Gene Sequence (5'→3') Product size Zebrafish primers for quantitative reverse transcription PCR notum1a CACTGACTGTGTGGACACCA (forward) 109 bp notum1a TCCTTCATAAGCCTGCCTGC (reverse) notum1b CCAACGTCACGGCCATGTTT (forward) 192 bp notum1b TGTCAATCAACTGCAGCGGG (reverse) dkk2 GCAGCAACTACATCTGCATTCC (forward) 150 bp dkk2 CTTCGTGACCTTTGAGAGAGATC (reverse) β-actin GAGAAGATCTGGCATCACAC (forward) 324 bp β-actin ATCAGGTAGTCTGTCAGGTC (reverse) Zebrafish primers for clone sequencing and in situ probe generation notum1a GGTGATGCTGGCTTTGGTTC (forward) 623 bp notum1a AGAGCAGATCCTTCACGACC (reverse) notum1b GCGGCTCTACACCAAAGACT (forward) 756 bp notum1b TGTCAATCAACTGCAGCGGG (reverse) notum1b AGGAGCTGCTGTGTGAGATG (forward 1) 1424 bp [full cDNA] notum1b TCTAGGTGCCGTTATTGAGC (reverse 1) 763 bp [cDNA 3' end] notum1b TGAACCTGGACCGTGTGTAT (forward 2) (with reverse 1) 770 bp [cDNA 5' end] notum1b CTGATCTGCTGATGATCCAG (reverse 2) (with forward 1) Zebrafish primers for CRISPR sgRNA generation notum1a_ex6 TAGGGTTCACTGATCATAAAGG (forward) notum1a_ex6