Effects of Atrazine on Testes in Xenopus Laevis Tadpoles and Juveniles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Disease and Clinical Signs Poster
African Clawed Frog Xenopus laevis Diseases and Clinical Signs in Photographs Therese Jones-Green University Biomedical Services Abstract Reported clinical signs associated with ill health and Clinical signs found between 2016 to 2018 Many diseases of Xenopus laevis frogs have been described in the • Opened in 2005, the biofacility can hold up to 900 female and 240 disease Clinical signs found between 2016 to 2018 male frogs in a Marine Biotech (now serviced by MBK Installations Ltd.) • 80 recirculating aquatic system. • Buoyancy problems 70 bridge this gap using photographs taken of frogs with clinical signs. The system monitors parameters such as: temperature, pH, • • Changes in activity e.g. lethargy or easily startled. My aims are: conductivity, and Total Gas Pressure. 60 • Unusual amount of skin shedding. 2018 • To share my experiences, via images, to help improve the welfare and • Frogs are maintained on mains fed water with a 20% water exchange. 50 ases • Weight loss, or failure to feed correctly. c husbandry of this species. • 2017 • Coelomic (body cavity) distention. 40 • To stimulate a dialogue which results in further sharing of ideas and 2016 Whole body bloat. 30 information. • Frogs are held under a 12 hour light/dark cycle. • Number of • Each tank has a PVC enrichment tube (30cm x 10cm), with smoothed • Fungal strands/tufts. 20 Introduction cut edges. • Sores or tremors at extremities (forearms and toes). 10 Between January 2016 and October 2018, cases of ill health and disease • Frogs are fed three times a week with a commercial trout pellet. • Redness or red spots. 0 Oedema Red leg Red leg (bad Red leg Gas bubble Sores on forearm Opaque cornea Skeletal Growth (cartilage Scar Cabletie Missing claws Eggbound in a colony of Xenopus laevis frogs housed by the University Biological hormone) (nematodes) disease deformity under skin) • The focus of the research undertaken in the biofacility is embryonic and • Excessive slime/mucous production or not enough (dry dull skin). -

Table S1. Nakharuthai and Srisapoome (2020)
Primer name Nucleotide sequence (5’→3’) Purpose CXC-1F New TGAACCCTGAGCTGAAGGCCGTGA Real-time PCR CXC-1R New TGAAGGTCTGATGAGTTTGTCGTC Real-time PCR CXC-1R CCTTCAGCTCAGGGTTCAAGC Genomic PCR CXC-2F New GCTTGAACCCCGAGCTGAAAAACG Real-time PCR CXC-2R New GTTCAGAGGTCGTATGAGGTGCTT Real-time PCR CXC-2F CAAGCAGGACAACAGTGTCTGTGT 3’RACE CXC-2AR GTTGCATGATTTGGATGCTGGGTAG 5’RACE CXC-1FSB AACATATGTCTCCCAGGCCCAACTCAAAC Southern blot CXC-1RSB CTCGAGTTATTTTGCACTGATGTGCAA Southern blot CXC1Exon1F CAAAGTGTTTCTGCTCCTGG Genomic PCR On-CXC1FOverEx CATATGCAACTCAAACAAGCAGGACAACAGT Overexpression On-CXC1ROverEx CTCGAGTTTTGCACTGATGTGCAATTTCAA Overexpression On-CXC2FOverEx CATATGCAACTCAAACAAGCAGGACAACAGT Overexpression On-CXC2ROverEx CTCGAGCATGGCAGCTGTGGAGGGTTCCAC Overexpression β-actinrealtimeF ACAGGATGCAGAAGGAGATCACAG Real-time PCR β-actinrealtimeR GTACTCCTGCTTGCTGATCCACAT Real-time PCR M13F AAAACGACGGCCAG Nucleotide sequencing M13R AACAGCTATGACCATG Nucleotide sequencing UPM-long (0.4 µM) CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT RACE PCR UPM-short (2 µM) CTAATAC GACTCACTATA GGGC RACE PCR Table S1. Nakharuthai and Srisapoome (2020) On-CXC1 Nucleotide (%) Amino acid (%) On-CXC2 Nucleotide (%) Amino acid (%) Versus identity identity similarity Versus identity identity Similarity Teleost fish 1. Rock bream, Oplegnathus fasciatus (AB703273) 64.5 49.1 68.1 O. fasciatus 70.7 57.7 75.4 2. Mandarin fish, Siniperca chuatsi (AAY79282) 63.2 48.1 68.9 S. chuatsi 70.5 54.0 78.8 3. Atlantic halibut, Hippoglossus hippoglossus (ACY54778) 52.0 39.3 51.1 H. hippoglossus 64.5 46.3 63.9 4. Common carp IL-8, Cyprinus carpio (ABE47600) 44.9 19.1 34.1 C. carpio 49.4 21.9 42.6 5. Rainbow trout IL-8, Oncorhynchus mykiss (CAC33585) 44.0 21.3 36.3 O. mykiss 47.3 23.7 44.4 6. Japanese flounder IL-8, Paralichthys olivaceus (AAL05442) 48.4 25.4 45.9 P. -

Finding Aid to the Historymakers® Video Oral History with Tyrone Hayes
Finding Aid to The HistoryMakers® Video Oral History with Tyrone Hayes Finding Aid to The HistoryMakers® Video Oral History with Tyrone Hayes Overview of the Collection Repository: The HistoryMakers®1900 S. Michigan Avenue Chicago, Illinois 60616 [email protected] www.thehistorymakers.com Creator: Hayes, Tyrone Title: The HistoryMakers® Video Oral History Interview with Tyrone Hayes, Dates: March 7, 2011 Bulk Dates: 2011 Physical Description: 20 MOV HD video files (3:06:51). Abstract: Biologist and biology professor Tyrone Hayes (1967 - ) is a leading researcher in amphibian endocrinology. Professor at the University of California, Berkeley, Hayes’s work has shown the herbicidal chemical Atrazine to act as an endocrine disruptor in the sexual development of frogs. Hayes was interviewed by The HistoryMakers® on March 7, 2011, in Berkeley, California. This collection is comprised of the original video footage of the interview. Identification: A2011_001 Language: The interview and records are in English. Biographical Note by The HistoryMakers® Biologist and biology professor Tyrone B. Hayes was born on July 29, 1967 in Columbia, South Carolina to Romeo and Susie Hayes. In the forests near Columbia, Hayes first became interested in the way that frogs morphed from tadpoles to their adult form. He graduated from Dreher High School in 1985 and then earned his B.A. and M.A. degrees in biology in 1989 from Harvard University. His dissertation on the genetic and environmental mechanisms determining the gender of the wood frog would be indicative of the research he would pursue later. After graduating from Harvard University, Hayes continued his studies at the University of California, Berkeley, where he received his Ph.D. -

Prof Tyrone Hayes 3577 Brunell Dr Oakland, CA 94602 USA Email: [email protected] Endocrine Disruptors and Early Developme
32 Prof Tyrone Hayes 3577 Brunell Dr Oakland, CA 94602 USA Email: [email protected] Endocrine disruptors and early development Tyrone B. Hayes* (University of California, Berkeley, CA, USA), WuQiang Fan, Toshihiko Yanase, Hidetaka Morinaga, Shigeki Gondo, Taijiro Okabe, and Masatoshi Nomura (Graduate School of Medical Science, Kyushu University, Fukuoka, Japan), Tomoko Komatsu, Ken-Ichirou Morohashi (Department of Developmental Biology, National Institute for Basic Biology, Okazaki, Japan), Ryoichi Takayanagi, and Hajime Nawata (Graduate School of Medical Science, Kyushu University, Fukuoka, Japan) Background and hypothesis: Atrazine is a potent endocrine disruptor that increases aromatase expression and estrogen production in some human cancer cell lines. The mechanism involves the inhibition of phosphodiesterase and subsequent elevation of cAMP. We hypothesized that the transcription factor (SF-1) is important for atrazine’s activity. Methodology: We compared SF-1 expression in atrazine responsive and non-responsive cell lines and transfected SF-1 into non-responsive cell lines to assess SF-1’s role in atrazine-induced aromatase. We used a luciferase reporter driven by the SF-1 dependent aromatase promoter (ArPII) to examine activation of this promoter by atrazine. We mutated the SF-1 binding site to confirm the role of SF-1. Finally, we examined atrazine and simazine’s ability to bind to SF-1 and enhance SF-1 binding to ArPII. Results: Atrazine-responsive adrenal carcinoma cells (H295R) expressed 54 times more SF-1 than non- responsive ovarian granulosa KGN cells. Exogenous SF-1 conveyed atrazine-responsiveness to otherwise non-responsive KGN and NIH/3T3 cells. Atrazine induced binding of SF-1 to chromatin and mutation of the SF-1 binding site in ArPII eliminated SF-1 binding and atrazine-responsiveness in H295R cells. -

Helical Insertion of Peptidoglycan Produces Chiral Ordering of the Bacterial Cell Wall
Helical insertion of peptidoglycan produces PNAS PLUS chiral ordering of the bacterial cell wall Siyuan Wanga,b, Leon Furchtgottc,d, Kerwyn Casey Huangd,1, and Joshua W. Shaevitza,e,1 aLewis-Sigler Institute for Integrative Genomics, Princeton University, Princeton, NJ 08544; bDepartment of Molecular Biology, Princeton University, Princeton, NJ 08544; cBiophysics Program, Harvard University, Cambridge, MA 02138; dDepartment of Bioengineering, Stanford University, Stanford, CA 94305; and eDepartment of Physics, Princeton University, Princeton, NJ 08854 AUTHOR SUMMARY Cells from all kingdoms of wall growth to demonstrate life face the task of that patterning of cell-wall constructing a specific, synthesis by left-handed mechanically robust three- MreB polymers leads to a dimensional (3D) cell shape right-handed chiral from molecular-scale organization of the glycan components. For many strands. This organization bacteria, maintaining a rigid, produces a left-handed rod-like shape facilitates a twisting of the cell body diverse range of behaviors during elongational growth. including swimming motility, We then confirm the detection of chemical existence of right-handed gradients, and nutrient access Fig. P1. Helical insertion of material into the bacterial cell wall (green) glycan organization in E. coli during elongational growth, guided by the protein MreB (yellow), leads and waste evacuation in by osmotically shocking biofilms. The static shape of to an emergent chiral order in the cell-wall network and twisting of the cell that can be visualized using surface-bound beads (red). surface-labeled cells and a bacterial cell is usually directly measuring the defined by the cell wall, a difference in stiffness macromolecular polymer network composed of glycan strands between the longitudinal and transverse directions. -

High Abundance of Invasive African Clawed Frog Xenopus Laevis in Chile: Challenges for Their Control and Updated Invasive Distribution
Management of Biological Invasions (2019) Volume 10, Issue 2: 377–388 CORRECTED PROOF Research Article High abundance of invasive African clawed frog Xenopus laevis in Chile: challenges for their control and updated invasive distribution Marta Mora1, Daniel J. Pons2,3, Alexandra Peñafiel-Ricaurte2, Mario Alvarado-Rybak2, Saulo Lebuy2 and Claudio Soto-Azat2,* 1Vida Nativa NGO, Santiago, Chile 2Centro de Investigación para la Sustentabilidad & Programa de Doctorado en Medicina de la Conservación, Facultad de Ciencias de la Vida, Universidad Andres Bello, Republica 440, Santiago, Chile 3Facultad de Ciencias Exactas, Departamento de Matemáticas, Universidad Andres Bello, Santiago, Republica 470, Santiago, Chile Author e-mails: [email protected] (CSA), [email protected] (MM), [email protected] (DJP), [email protected] (APR), [email protected] (MAR), [email protected] (SL) *Corresponding author Citation: Mora M, Pons DJ, Peñafiel- Ricaurte A, Alvarado-Rybak M, Lebuy S, Abstract Soto-Azat C (2019) High abundance of invasive African clawed frog Xenopus Invasive African clawed frog Xenopus laevis (Daudin, 1802) are considered a major laevis in Chile: challenges for their control threat to aquatic environments. Beginning in the early 1970s, invasive populations and updated invasive distribution. have now been established throughout much of central Chile. Between September Management of Biological Invasions 10(2): and December 2015, we studied a population of X. laevis from a small pond in 377–388, https://doi.org/10.3391/mbi.2019. 10.2.11 Viña del Mar, where we estimated the population size and evaluated the use of hand nets as a method of control. First, by means of a non-linear extrapolation Received: 26 October 2018 model using the data from a single capture session of 200 min, a population size of Accepted: 7 March 2019 1,182 post-metamorphic frogs (range: 1,168–1,195 [quadratic error]) and a density Published: 16 May 2019 of 13.7 frogs/m2 (range: 13.6–13.9) of surface water were estimated. -
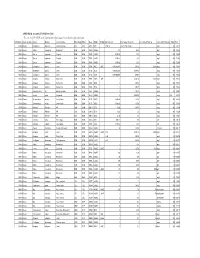
APPENDIX K: Accepted ECOTOX Data Table
APPENDIX K: Accepted ECOTOX Data Table The code list for ECOTOX can be found at: http://cfpub.epa.gov/ecotox/blackbox/help/codelist.pdf CAS Number Chemical Name Genus Species Common Name Effect Group Effect Meas Endpt1 Endpt2 Dur Preferred Conc Value1 Preferred Conc Value2 Preferred Conc Units Preferred % Purity Ref # 330541 Diuron Pimephales promelas Fathead minnow ACC ACC GACC BCF 1 TO 24 0.00315 TO 0.0304 mg/L 100 12612 330541 Diuron Rattus norvegicus Norway rat BCM BCM PHPH NOAEL 140 2500 ppm 100 90555 330541 Diuron Zostera capricorni Eelgrass BCM BCM FLRS LOAEL 8.33E-02 0.01 mg/L 100 72996 330541 Diuron Zostera capricorni Eelgrass BCM BCM FLRS LOAEL 8.33E-02 0.01 mg/L 100 72996 330541 Diuron Zostera capricorni Eelgrass BCM BCM FLRS LOAEL 8.33E-02 0.01 mg/L 100 72996 330541 Diuron Seriatopora hystrix Coral BCM BCM FLRS NOEC LOEC 0.416666667 0.0003 0.0003 mg/L 100 75334 330541 Diuron Seriatopora hystrix Coral BCM BCM FLRS EC25 0.416666667 0.00085 mg/L 100 75334 330541 Diuron Seriatopora hystrix Coral BCM BCM FLRS EC50 0.416666667 0.0023 mg/L 100 75334 330541 Diuron Acropora formosa Stony coral BCM BCM FLRS NOEC LOEC 1 0.00003 0.0003 mg/L 100 75334 330541 Diuron Acropora formosa Stony coral BCM BCM FLRS EC25 1 0.0012 mg/L 100 75334 330541 Diuron Acropora formosa Stony coral BCM BCM FLRS EC50 1 0.0027 mg/L 100 75334 330541 Diuron Synechococcus sp. Blue-green algae BCM BCM CHCT NOAEL 3 0.0033 mg/L 99 98904 330541 Diuron Lemna minor Duckweed BCM BCM GLTH LOAEL 2 0.02475 mg/L >99 64164 330541 Diuron Scenedesmus acutus Green Algae BCM BCM -

Xenopus Laevis
Guidance on the housing and care of the African clawed frog Xenopus laevis Barney T Reed Research Animals Department - RSPCA Guidelines for the housing and care of the African clawed frog (Xenopus laevis) May 2005 Guidance on the housing and care of the African clawed frog Xenopus laevis) Acknowledgements The author would like to sincerely thank the following people for their helpful and constructive comments during the preparation of this report: Mr. D. Anderson - Home Office, Animals Scientific Procedures Division Mr. M. Brown - MRC Laboratory of Molecular Biology, Cambridge Dr. G. Griffin - Canadian Council for Animal Care (CCAC) Dr. M. Guille - School of Biological Sciences, University of Portsmouth Dr. P. Hawkins - Research Animals Department, RSPCA Dr. R. Hubrecht - Universities Federation for Animal Welfare (UFAW) Dr. M. Jennings - Research Animals Department, RSPCA Dr. K. Mathers - MRC National Institute for Medical Research Dr. G. Sanders - School of Medicine, University of Washington Prof. R. Tinsley - School of Biological Sciences, University of Bristol Special thanks also to those organisations whose establishments I visited and whose scientific and animal care staff provided valuable input and advice. Note: The views expressed in this document are those of the author, and may not necessarily represent those of the persons named above or their affiliated organisations. About the author Barney Reed studied psychology and biology at the University of Exeter before obtaining a MSc in applied animal behaviour and animal welfare from the University of Edinburgh. He worked in the Animals Scientific Procedures Division of the Home Office before joining the research animals department of the RSPCA as a scientific officer. -

Danio Rerio) Notum1b, Notum1a, and Notum2; Fruit Fly (Drosophila Melanogaster) Notum; and African Clawed Frog (Xenopus Laevis) Notum
Figure S1: Protein alignments of NOTUM homologs. Amino acid alignments of: human (Homo sapien) NOTUM; zebrafish (Danio rerio) notum1b, notum1a, and notum2; fruit fly (Drosophila melanogaster) notum; and African clawed frog (Xenopus laevis) notum. Note the high conservation around the active site motif G-X-S-X-G (blue box) and the Ser, Asp, His catalytic triad (red boxes) characteristic of the α/β hydrolase protein family. Table S1: Oligonucleotides utilized for qRT-PCR, clone sequencing, ISH, sgRNA synthesis, and genotyping. Gene Sequence (5'→3') Product size Zebrafish primers for quantitative reverse transcription PCR notum1a CACTGACTGTGTGGACACCA (forward) 109 bp notum1a TCCTTCATAAGCCTGCCTGC (reverse) notum1b CCAACGTCACGGCCATGTTT (forward) 192 bp notum1b TGTCAATCAACTGCAGCGGG (reverse) dkk2 GCAGCAACTACATCTGCATTCC (forward) 150 bp dkk2 CTTCGTGACCTTTGAGAGAGATC (reverse) β-actin GAGAAGATCTGGCATCACAC (forward) 324 bp β-actin ATCAGGTAGTCTGTCAGGTC (reverse) Zebrafish primers for clone sequencing and in situ probe generation notum1a GGTGATGCTGGCTTTGGTTC (forward) 623 bp notum1a AGAGCAGATCCTTCACGACC (reverse) notum1b GCGGCTCTACACCAAAGACT (forward) 756 bp notum1b TGTCAATCAACTGCAGCGGG (reverse) notum1b AGGAGCTGCTGTGTGAGATG (forward 1) 1424 bp [full cDNA] notum1b TCTAGGTGCCGTTATTGAGC (reverse 1) 763 bp [cDNA 3' end] notum1b TGAACCTGGACCGTGTGTAT (forward 2) (with reverse 1) 770 bp [cDNA 5' end] notum1b CTGATCTGCTGATGATCCAG (reverse 2) (with forward 1) Zebrafish primers for CRISPR sgRNA generation notum1a_ex6 TAGGGTTCACTGATCATAAAGG (forward) notum1a_ex6 -

Atrazine and Amphibians: a Story of Profits, Controversy, and Animus
This article was originally published in the Encyclopedia of the Anthropocene published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author’s institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who you know, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial Rohr J.R. (2018) Atrazine and Amphibians: A Story of Profits, Controversy, and Animus. In: Dominick A. DellaSala, and Michael I. Goldstein (eds.) The Encyclopedia of the Anthropocene, vol. 5, p. 141-148. Oxford: Elsevier. © 2018 Elsevier Inc. All rights reserved. Author's personal copy Atrazine and Amphibians: A Story of Profits, Controversy, and Animus JR Rohr, University of South Florida, Tampa, FL, United States © 2018 Elsevier Inc. All rights reserved. Introduction The herbicide atrazine (2-chloro-4-(ethylamino)-6-(isopropylamino)-S-triazine) is one of the most widely studied, commonly used, and controversial pesticides on the planet. A search for the term “atrazine” in the search engine Web of Science (conducted on 11/17/2016) produced 11,203 studies. Atrazine was the most commonly used pesticide in the United States before it was recently surpassed by the herbicide glyphosate (Roundup®), which happened because of the advent of genetically modified crops (Grube et al., 2011). -
![Gastrulation in Mus Musculus (Common House Mouse) [1]](https://docslib.b-cdn.net/cover/0047/gastrulation-in-mus-musculus-common-house-mouse-1-2460047.webp)
Gastrulation in Mus Musculus (Common House Mouse) [1]
Published on The Embryo Project Encyclopedia (https://embryo.asu.edu) Gastrulation in Mus musculus (common house mouse) [1] By: Wolter, Justin M. Keywords: Model organisms [2] Mice [3] Gastrulation [4] Germ layers [5] As mice embryos develop, they undergo a stage of development calledg astrulation [7]. The hallmark of vertebrate gastrulation [7] is the reorganization of the inner cell mass [8] (ICM) into the three germ layers [9]: ectoderm [10], mesoderm [11], and endoderm [12]. Mammalian embryogenesis [13] occurs within organisms; therefore, gastrulation [7] was originally described in species with easily observable embryos. For example, the African clawed frog [14] (Xenopus laevis [15]) is a widely used organism to study gastrulation [7] because the large embryos develop inside a translucent membrane. Domestic chickens G( allus gallus) provided researchers another early model to study gastrulation [7] because researchers could open the egg [16] during development to look inside. Despite the challenges associated with studying mammalian gastrulation [7], the common house mouse [17] (Mus musculus) has helped to shed light on the unique adaptations associated with mammalian development. Gastrulation in the mouse [6] begins shortly after a blastula [18] implants into the uterine wall of the mother, and is immediately followed by the development of the various organ systems (organogenesis [19]). This coordinated movement of cells results in a spatially organized embryo, and assembles the framework upon which future developmental processes will build the body. The term for an embryo undergoing gastrulation [7] is the gastrula [20], a term coined by Ernst Haeckel [21] Germany in 1872, and expanded upon in his 1874 Studien zur Gastraea-theorie (Studies for the Gastrea Theory). -

Introduction
introduction Harvard Apparatus has been supplying life science researchers studying these model organisms have been making with innovative products and excellent customer support since considerable contributions using Harvard Apparatus products 1902. Over the last 102 years Harvard Apparatus has played a for years, but now new products are required to enhance their significant role in the advancement of science, and we are research. For this catalog, Harvard Apparatus reviewed proud to maintain that role today. Harvard Apparatus is well published research on model organisms and organized our known for its support of small animal research, providing products accordingly. We have added new products to enhance products ideal for studies on mice, rats, guinea pigs, cats and model organism research and included sample publications to dogs. Now the time has come to extend our renowned support assist you in utilizing both the new and existing products. to new research models in bioresearch, including Drosophila, Nematodes, Xenopus and Zebrafish. Life science researchers What are 'model organisms'? (Richard Twyman) A model organism is a species that has been widely studied, usually because it is easy to maintain and breed in a laboratory setting and has particular experimental advantages. Over the years, a great deal of data has accumulated about such organisms and this in itself makes them more attractive to study. Model organisms are used to obtain information about other species – including humans – that are more difficult to study directly. MODEL TYPES SPECIES CHARACTERISTICS Genetic Model Organisms These species are amenable to Baker’s Yeast Many different mutants are generally available and genetic analysis: (Saccharomyces cerevisiae) highly detailed genetic maps can be created.