Stereocenter
Top View
- Organocatalysis and Beyond
- Review of Stereochemistry
- Solvent Engineering and Other Reaction Design Methods for Favouring Enzyme- Catalysed Synthesis
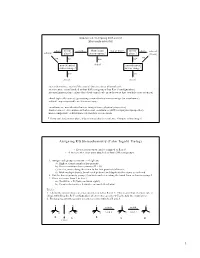
- Stereochemistry H O H Step 1
- CHM 204—Organic Chemistry Introduction to Stereochemistry
- Recent Advances in the Catalytic Asymmetric Reactions of Oxaziridines
- Enantioselective Synthesis of Acyclic Compounds
- 1 CHAPTER 1 Introduction to Enantioselective Oxidation Chemistry 1.1 Oxidation in Biological Systems the Selective Oxidation Of
- Streochemistry Stereochemistry Deals with Three-Dimensional
- Separation of Enantiomers by Chromatography As a Vehicle for Chiral Catalysis
- Science, 369, 1113
- Stereochemistry
- CALB Immobilized Onto Magnetic Nanoparticles for Efficient Kinetic
- Eighth Southeast Enzyme Conference
- Tutorial on Stereochemistry
- 2.01 R,S Nomenclature Answers.Cdx
- Asymmetric Organocatalysis
- The Stereocenter