Energy level
Top View
- Thermal and Residual Excited-State Population in a 3D Transmon Qubit
- 12 Wavefunction Collapse
- The Transmon Qubit
- Energy Level Diagrams
- Dirac Generalized Relativistic Quantum Wave Function Which Gives Right Electrons Number in Each Energy Level by Controlling Quan
- Can Wavefunction Collapse Conserve Energy?
- 13 • Electron Configurations Electron Energy Levels
- Chapter 4 Time–Independent Schrödinger Equation
- Statistical Thermodynamics
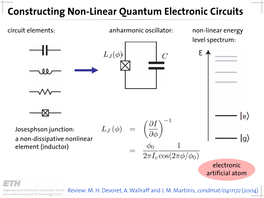
- A 4 Superconducting Qubits
- The Schrödinger Wave Equation
- Lecture for Bob
- Statistical Physics
- Interpreting Quantum Mechanics in Terms of Random Discontinuous Motion of Particles
- History and Some Aspects of the Lamb Shift
- 1 Outlook and High Level Overview
- Bohr Theory in the Atomic Physics
- Figure 33: the First Three Wavefunctions for a Particle Moving in One Dimension Inside a Box with Walls at X=0 and X=L. Must