Statistical Physics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Part 5: the Bose-Einstein Distribution
PHYS393 – Statistical Physics Part 5: The Bose-Einstein Distribution Distinguishable and indistinguishable particles In the previous parts of this course, we derived the Boltzmann distribution, which described how the number of distinguishable particles in different energy states varied with the energy of those states, at different temperatures: N εj nj = e− kT . (1) Z However, in systems consisting of collections of identical fermions or identical bosons, the wave function of the system has to be either antisymmetric (for fermions) or symmetric (for bosons) under interchange of any two particles. With the allowed wave functions, it is no longer possible to identify a particular particle with a particular energy state. Instead, all the particles are “shared” between the occupied states. The particles are said to be indistinguishable . Statistical Physics 1 Part 5: The Bose-Einstein Distribution Indistinguishable fermions In the case of indistinguishable fermions, the wave function for the overall system must be antisymmetric under the interchange of any two particles. One consequence of this is the Pauli exclusion principle: any given state can be occupied by at most one particle (though we can’t say which particle!) For example, for a system of two fermions, a possible wave function might be: 1 ψ(x1, x 2) = [ψ (x1)ψ (x2) ψ (x2)ψ (x1)] . (2) √2 A B − A B Here, x1 and x2 are the coordinates of the two particles, and A and B are the two occupied states. If we try to put the two particles into the same state, then the wave function vanishes. Statistical Physics 2 Part 5: The Bose-Einstein Distribution Indistinguishable fermions Finding the distribution with the maximum number of microstates for a system of identical fermions leads to the Fermi-Dirac distribution: g n = i . -

Key Concepts for Future QIS Learners Workshop Output Published Online May 13, 2020
Key Concepts for Future QIS Learners Workshop output published online May 13, 2020 Background and Overview On behalf of the Interagency Working Group on Workforce, Industry and Infrastructure, under the NSTC Subcommittee on Quantum Information Science (QIS), the National Science Foundation invited 25 researchers and educators to come together to deliberate on defining a core set of key concepts for future QIS learners that could provide a starting point for further curricular and educator development activities. The deliberative group included university and industry researchers, secondary school and college educators, and representatives from educational and professional organizations. The workshop participants focused on identifying concepts that could, with additional supporting resources, help prepare secondary school students to engage with QIS and provide possible pathways for broader public engagement. This workshop report identifies a set of nine Key Concepts. Each Concept is introduced with a concise overall statement, followed by a few important fundamentals. Connections to current and future technologies are included, providing relevance and context. The first Key Concept defines the field as a whole. Concepts 2-6 introduce ideas that are necessary for building an understanding of quantum information science and its applications. Concepts 7-9 provide short explanations of critical areas of study within QIS: quantum computing, quantum communication and quantum sensing. The Key Concepts are not intended to be an introductory guide to quantum information science, but rather provide a framework for future expansion and adaptation for students at different levels in computer science, mathematics, physics, and chemistry courses. As such, it is expected that educators and other community stakeholders may not yet have a working knowledge of content covered in the Key Concepts. -

Unit 1 Old Quantum Theory
UNIT 1 OLD QUANTUM THEORY Structure Introduction Objectives li;,:overy of Sub-atomic Particles Earlier Atom Models Light as clectromagnetic Wave Failures of Classical Physics Black Body Radiation '1 Heat Capacity Variation Photoelectric Effect Atomic Spectra Planck's Quantum Theory, Black Body ~diation. and Heat Capacity Variation Einstein's Theory of Photoelectric Effect Bohr Atom Model Calculation of Radius of Orbits Energy of an Electron in an Orbit Atomic Spectra and Bohr's Theory Critical Analysis of Bohr's Theory Refinements in the Atomic Spectra The61-y Summary Terminal Questions Answers 1.1 INTRODUCTION The ideas of classical mechanics developed by Galileo, Kepler and Newton, when applied to atomic and molecular systems were found to be inadequate. Need was felt for a theory to describe, correlate and predict the behaviour of the sub-atomic particles. The quantum theory, proposed by Max Planck and applied by Einstein and Bohr to explain different aspects of behaviour of matter, is an important milestone in the formulation of the modern concept of atom. In this unit, we will study how black body radiation, heat capacity variation, photoelectric effect and atomic spectra of hydrogen can be explained on the basis of theories proposed by Max Planck, Einstein and Bohr. They based their theories on the postulate that all interactions between matter and radiation occur in terms of definite packets of energy, known as quanta. Their ideas, when extended further, led to the evolution of wave mechanics, which shows the dual nature of matter -

Classical Mechanics
Classical Mechanics Hyoungsoon Choi Spring, 2014 Contents 1 Introduction4 1.1 Kinematics and Kinetics . .5 1.2 Kinematics: Watching Wallace and Gromit ............6 1.3 Inertia and Inertial Frame . .8 2 Newton's Laws of Motion 10 2.1 The First Law: The Law of Inertia . 10 2.2 The Second Law: The Equation of Motion . 11 2.3 The Third Law: The Law of Action and Reaction . 12 3 Laws of Conservation 14 3.1 Conservation of Momentum . 14 3.2 Conservation of Angular Momentum . 15 3.3 Conservation of Energy . 17 3.3.1 Kinetic energy . 17 3.3.2 Potential energy . 18 3.3.3 Mechanical energy conservation . 19 4 Solving Equation of Motions 20 4.1 Force-Free Motion . 21 4.2 Constant Force Motion . 22 4.2.1 Constant force motion in one dimension . 22 4.2.2 Constant force motion in two dimensions . 23 4.3 Varying Force Motion . 25 4.3.1 Drag force . 25 4.3.2 Harmonic oscillator . 29 5 Lagrangian Mechanics 30 5.1 Configuration Space . 30 5.2 Lagrangian Equations of Motion . 32 5.3 Generalized Coordinates . 34 5.4 Lagrangian Mechanics . 36 5.5 D'Alembert's Principle . 37 5.6 Conjugate Variables . 39 1 CONTENTS 2 6 Hamiltonian Mechanics 40 6.1 Legendre Transformation: From Lagrangian to Hamiltonian . 40 6.2 Hamilton's Equations . 41 6.3 Configuration Space and Phase Space . 43 6.4 Hamiltonian and Energy . 45 7 Central Force Motion 47 7.1 Conservation Laws in Central Force Field . 47 7.2 The Path Equation . -

Canonical Ensemble
ME346A Introduction to Statistical Mechanics { Wei Cai { Stanford University { Win 2011 Handout 8. Canonical Ensemble January 26, 2011 Contents Outline • In this chapter, we will establish the equilibrium statistical distribution for systems maintained at a constant temperature T , through thermal contact with a heat bath. • The resulting distribution is also called Boltzmann's distribution. • The canonical distribution also leads to definition of the partition function and an expression for Helmholtz free energy, analogous to Boltzmann's Entropy formula. • We will study energy fluctuation at constant temperature, and witness another fluctuation- dissipation theorem (FDT) and finally establish the equivalence of micro canonical ensemble and canonical ensemble in the thermodynamic limit. (We first met a mani- festation of FDT in diffusion as Einstein's relation.) Reading Assignment: Reif x6.1-6.7, x6.10 1 1 Temperature For an isolated system, with fixed N { number of particles, V { volume, E { total energy, it is most conveniently described by the microcanonical (NVE) ensemble, which is a uniform distribution between two constant energy surfaces. const E ≤ H(fq g; fp g) ≤ E + ∆E ρ (fq g; fp g) = i i (1) mc i i 0 otherwise Statistical mechanics also provides the expression for entropy S(N; V; E) = kB ln Ω. In thermodynamics, S(N; V; E) can be transformed to a more convenient form (by Legendre transform) of Helmholtz free energy A(N; V; T ), which correspond to a system with constant N; V and temperature T . Q: Does the transformation from N; V; E to N; V; T have a meaning in statistical mechanics? A: The ensemble of systems all at constant N; V; T is called the canonical NVT ensemble. -

Magnetism, Magnetic Properties, Magnetochemistry
Magnetism, Magnetic Properties, Magnetochemistry 1 Magnetism All matter is electronic Positive/negative charges - bound by Coulombic forces Result of electric field E between charges, electric dipole Electric and magnetic fields = the electromagnetic interaction (Oersted, Maxwell) Electric field = electric +/ charges, electric dipole Magnetic field ??No source?? No magnetic charges, N-S No magnetic monopole Magnetic field = motion of electric charges (electric current, atomic motions) Magnetic dipole – magnetic moment = i A [A m2] 2 Electromagnetic Fields 3 Magnetism Magnetic field = motion of electric charges • Macro - electric current • Micro - spin + orbital momentum Ampère 1822 Poisson model Magnetic dipole – magnetic (dipole) moment [A m2] i A 4 Ampere model Magnetism Microscopic explanation of source of magnetism = Fundamental quantum magnets Unpaired electrons = spins (Bohr 1913) Atomic building blocks (protons, neutrons and electrons = fermions) possess an intrinsic magnetic moment Relativistic quantum theory (P. Dirac 1928) SPIN (quantum property ~ rotation of charged particles) Spin (½ for all fermions) gives rise to a magnetic moment 5 Atomic Motions of Electric Charges The origins for the magnetic moment of a free atom Motions of Electric Charges: 1) The spins of the electrons S. Unpaired spins give a paramagnetic contribution. Paired spins give a diamagnetic contribution. 2) The orbital angular momentum L of the electrons about the nucleus, degenerate orbitals, paramagnetic contribution. The change in the orbital moment -

Quantum Theory of the Hydrogen Atom
Quantum Theory of the Hydrogen Atom Chemistry 35 Fall 2000 Balmer and the Hydrogen Spectrum n 1885: Johann Balmer, a Swiss schoolteacher, empirically deduced a formula which predicted the wavelengths of emission for Hydrogen: l (in Å) = 3645.6 x n2 for n = 3, 4, 5, 6 n2 -4 •Predicts the wavelengths of the 4 visible emission lines from Hydrogen (which are called the Balmer Series) •Implies that there is some underlying order in the atom that results in this deceptively simple equation. 2 1 The Bohr Atom n 1913: Niels Bohr uses quantum theory to explain the origin of the line spectrum of hydrogen 1. The electron in a hydrogen atom can exist only in discrete orbits 2. The orbits are circular paths about the nucleus at varying radii 3. Each orbit corresponds to a particular energy 4. Orbit energies increase with increasing radii 5. The lowest energy orbit is called the ground state 6. After absorbing energy, the e- jumps to a higher energy orbit (an excited state) 7. When the e- drops down to a lower energy orbit, the energy lost can be given off as a quantum of light 8. The energy of the photon emitted is equal to the difference in energies of the two orbits involved 3 Mohr Bohr n Mathematically, Bohr equated the two forces acting on the orbiting electron: coulombic attraction = centrifugal accelleration 2 2 2 -(Z/4peo)(e /r ) = m(v /r) n Rearranging and making the wild assumption: mvr = n(h/2p) n e- angular momentum can only have certain quantified values in whole multiples of h/2p 4 2 Hydrogen Energy Levels n Based on this model, Bohr arrived at a simple equation to calculate the electron energy levels in hydrogen: 2 En = -RH(1/n ) for n = 1, 2, 3, 4, . -

Forced Mechanical Oscillations
169 Carl von Ossietzky Universität Oldenburg – Faculty V - Institute of Physics Module Introductory laboratory course physics – Part I Forced mechanical oscillations Keywords: HOOKE's law, harmonic oscillation, harmonic oscillator, eigenfrequency, damped harmonic oscillator, resonance, amplitude resonance, energy resonance, resonance curves References: /1/ DEMTRÖDER, W.: „Experimentalphysik 1 – Mechanik und Wärme“, Springer-Verlag, Berlin among others. /2/ TIPLER, P.A.: „Physik“, Spektrum Akademischer Verlag, Heidelberg among others. /3/ ALONSO, M., FINN, E. J.: „Fundamental University Physics, Vol. 1: Mechanics“, Addison-Wesley Publishing Company, Reading (Mass.) among others. 1 Introduction It is the object of this experiment to study the properties of a „harmonic oscillator“ in a simple mechanical model. Such harmonic oscillators will be encountered in different fields of physics again and again, for example in electrodynamics (see experiment on electromagnetic resonant circuit) and atomic physics. Therefore it is very important to understand this experiment, especially the importance of the amplitude resonance and phase curves. 2 Theory 2.1 Undamped harmonic oscillator Let us observe a set-up according to Fig. 1, where a sphere of mass mK is vertically suspended (x-direc- tion) on a spring. Let us neglect the effects of friction for the moment. When the sphere is at rest, there is an equilibrium between the force of gravity, which points downwards, and the dragging resilience which points upwards; the centre of the sphere is then in the position x = 0. A deflection of the sphere from its equilibrium position by x causes a proportional dragging force FR opposite to x: (1) FxR ∝− The proportionality constant (elastic or spring constant or directional quantity) is denoted D, and Eq. -

Identical Particles
8.06 Spring 2016 Lecture Notes 4. Identical particles Aram Harrow Last updated: May 19, 2016 Contents 1 Fermions and Bosons 1 1.1 Introduction and two-particle systems .......................... 1 1.2 N particles ......................................... 3 1.3 Non-interacting particles .................................. 5 1.4 Non-zero temperature ................................... 7 1.5 Composite particles .................................... 7 1.6 Emergence of distinguishability .............................. 9 2 Degenerate Fermi gas 10 2.1 Electrons in a box ..................................... 10 2.2 White dwarves ....................................... 12 2.3 Electrons in a periodic potential ............................. 16 3 Charged particles in a magnetic field 21 3.1 The Pauli Hamiltonian ................................... 21 3.2 Landau levels ........................................ 23 3.3 The de Haas-van Alphen effect .............................. 24 3.4 Integer Quantum Hall Effect ............................... 27 3.5 Aharonov-Bohm Effect ................................... 33 1 Fermions and Bosons 1.1 Introduction and two-particle systems Previously we have discussed multiple-particle systems using the tensor-product formalism (cf. Section 1.2 of Chapter 3 of these notes). But this applies only to distinguishable particles. In reality, all known particles are indistinguishable. In the coming lectures, we will explore the mathematical and physical consequences of this. First, consider classical many-particle systems. If a single particle has state described by position and momentum (~r; p~), then the state of N distinguishable particles can be written as (~r1; p~1; ~r2; p~2;:::; ~rN ; p~N ). The notation (·; ·;:::; ·) denotes an ordered list, in which different posi tions have different meanings; e.g. in general (~r1; p~1; ~r2; p~2)6 = (~r2; p~2; ~r1; p~1). 1 To describe indistinguishable particles, we can use set notation. -
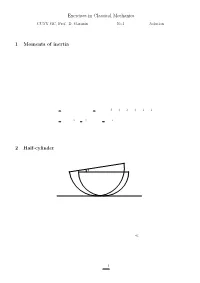
Exercises in Classical Mechanics 1 Moments of Inertia 2 Half-Cylinder
Exercises in Classical Mechanics CUNY GC, Prof. D. Garanin No.1 Solution ||||||||||||||||||||||||||||||||||||||||| 1 Moments of inertia (5 points) Calculate tensors of inertia with respect to the principal axes of the following bodies: a) Hollow sphere of mass M and radius R: b) Cone of the height h and radius of the base R; both with respect to the apex and to the center of mass. c) Body of a box shape with sides a; b; and c: Solution: a) By symmetry for the sphere I®¯ = I±®¯: (1) One can ¯nd I easily noticing that for the hollow sphere is 1 1 X ³ ´ I = (I + I + I ) = m y2 + z2 + z2 + x2 + x2 + y2 3 xx yy zz 3 i i i i i i i i 2 X 2 X 2 = m r2 = R2 m = MR2: (2) 3 i i 3 i 3 i i b) and c): Standard solutions 2 Half-cylinder ϕ (10 points) Consider a half-cylinder of mass M and radius R on a horizontal plane. a) Find the position of its center of mass (CM) and the moment of inertia with respect to CM. b) Write down the Lagrange function in terms of the angle ' (see Fig.) c) Find the frequency of cylinder's oscillations in the linear regime, ' ¿ 1. Solution: (a) First we ¯nd the distance a between the CM and the geometrical center of the cylinder. With σ being the density for the cross-sectional surface, so that ¼R2 M = σ ; (3) 2 1 one obtains Z Z 2 2σ R p σ R q a = dx x R2 ¡ x2 = dy R2 ¡ y M 0 M 0 ¯ 2 ³ ´3=2¯R σ 2 2 ¯ σ 2 3 4 = ¡ R ¡ y ¯ = R = R: (4) M 3 0 M 3 3¼ The moment of inertia of the half-cylinder with respect to the geometrical center is the same as that of the cylinder, 1 I0 = MR2: (5) 2 The moment of inertia with respect to the CM I can be found from the relation I0 = I + Ma2 (6) that yields " µ ¶ # " # 1 4 2 1 32 I = I0 ¡ Ma2 = MR2 ¡ = MR2 1 ¡ ' 0:3199MR2: (7) 2 3¼ 2 (3¼)2 (b) The Lagrange function L is given by L('; '_ ) = T ('; '_ ) ¡ U('): (8) The potential energy U of the half-cylinder is due to the elevation of its CM resulting from the deviation of ' from zero. -

Further Quantum Physics
Further Quantum Physics Concepts in quantum physics and the structure of hydrogen and helium atoms Prof Andrew Steane January 18, 2005 2 Contents 1 Introduction 7 1.1 Quantum physics and atoms . 7 1.1.1 The role of classical and quantum mechanics . 9 1.2 Atomic physics—some preliminaries . .... 9 1.2.1 Textbooks...................................... 10 2 The 1-dimensional projectile: an example for revision 11 2.1 Classicaltreatment................................. ..... 11 2.2 Quantum treatment . 13 2.2.1 Mainfeatures..................................... 13 2.2.2 Precise quantum analysis . 13 3 Hydrogen 17 3.1 Some semi-classical estimates . 17 3.2 2-body system: reduced mass . 18 3.2.1 Reduced mass in quantum 2-body problem . 19 3.3 Solution of Schr¨odinger equation for hydrogen . ..... 20 3.3.1 General features of the radial solution . 21 3.3.2 Precisesolution.................................. 21 3.3.3 Meanradius...................................... 25 3.3.4 How to remember hydrogen . 25 3.3.5 Mainpoints.................................... 25 3.3.6 Appendix on series solution of hydrogen equation, off syllabus . 26 3 4 CONTENTS 4 Hydrogen-like systems and spectra 27 4.1 Hydrogen-like systems . 27 4.2 Spectroscopy ........................................ 29 4.2.1 Main points for use of grating spectrograph . ...... 29 4.2.2 Resolution...................................... 30 4.2.3 Usefulness of both emission and absorption methods . 30 4.3 The spectrum for hydrogen . 31 5 Introduction to fine structure and spin 33 5.1 Experimental observation of fine structure . ..... 33 5.2 TheDiracresult ..................................... 34 5.3 Schr¨odinger method to account for fine structure . 35 5.4 Physical nature of orbital and spin angular momenta . -

The Emergence of a Classical World in Bohmian Mechanics
The Emergence of a Classical World in Bohmian Mechanics Davide Romano Lausanne, 02 May 2014 Structure of QM • Physical state of a quantum system: - (normalized) vector in Hilbert space: state-vector - Wavefunction: the state-vector expressed in the coordinate representation Structure of QM • Physical state of a quantum system: - (normalized) vector in Hilbert space: state-vector; - Wavefunction: the state-vector expressed in the coordinate representation; • Physical properties of the system (Observables): - Observable ↔ hermitian operator acting on the state-vector or the wavefunction; - Given a certain operator A, the quantum system has a definite physical property respect to A iff it is an eigenstate of A; - The only possible measurement’s results of the physical property corresponding to the operator A are the eigenvalues of A; - Why hermitian operators? They have a real spectrum of eigenvalues → the result of a measurement can be interpreted as a physical value. Structure of QM • In the general case, the state-vector of a system is expressed as a linear combination of the eigenstates of a generic operator that acts upon it: 퐴 Ψ = 푎 Ψ1 + 푏|Ψ2 • When we perform a measure of A on the system |Ψ , the latter randomly collapses or 2 2 in the state |Ψ1 with probability |푎| or in the state |Ψ2 with probability |푏| . Structure of QM • In the general case, the state-vector of a system is expressed as a linear combination of the eigenstates of a generic operator that acts upon it: 퐴 Ψ = 푎 Ψ1 + 푏|Ψ2 • When we perform a measure of A on the system |Ψ , the latter randomly collapses or 2 2 in the state |Ψ1 with probability |푎| or in the state |Ψ2 with probability |푏| .