The Schrödinger Wave Equation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Path Integrals in Quantum Mechanics
Path Integrals in Quantum Mechanics Dennis V. Perepelitsa MIT Department of Physics 70 Amherst Ave. Cambridge, MA 02142 Abstract We present the path integral formulation of quantum mechanics and demon- strate its equivalence to the Schr¨odinger picture. We apply the method to the free particle and quantum harmonic oscillator, investigate the Euclidean path integral, and discuss other applications. 1 Introduction A fundamental question in quantum mechanics is how does the state of a particle evolve with time? That is, the determination the time-evolution ψ(t) of some initial | i state ψ(t ) . Quantum mechanics is fully predictive [3] in the sense that initial | 0 i conditions and knowledge of the potential occupied by the particle is enough to fully specify the state of the particle for all future times.1 In the early twentieth century, Erwin Schr¨odinger derived an equation specifies how the instantaneous change in the wavefunction d ψ(t) depends on the system dt | i inhabited by the state in the form of the Hamiltonian. In this formulation, the eigenstates of the Hamiltonian play an important role, since their time-evolution is easy to calculate (i.e. they are stationary). A well-established method of solution, after the entire eigenspectrum of Hˆ is known, is to decompose the initial state into this eigenbasis, apply time evolution to each and then reassemble the eigenstates. That is, 1In the analysis below, we consider only the position of a particle, and not any other quantum property such as spin. 2 D.V. Perepelitsa n=∞ ψ(t) = exp [ iE t/~] n ψ(t ) n (1) | i − n h | 0 i| i n=0 X This (Hamiltonian) formulation works in many cases. -

Key Concepts for Future QIS Learners Workshop Output Published Online May 13, 2020
Key Concepts for Future QIS Learners Workshop output published online May 13, 2020 Background and Overview On behalf of the Interagency Working Group on Workforce, Industry and Infrastructure, under the NSTC Subcommittee on Quantum Information Science (QIS), the National Science Foundation invited 25 researchers and educators to come together to deliberate on defining a core set of key concepts for future QIS learners that could provide a starting point for further curricular and educator development activities. The deliberative group included university and industry researchers, secondary school and college educators, and representatives from educational and professional organizations. The workshop participants focused on identifying concepts that could, with additional supporting resources, help prepare secondary school students to engage with QIS and provide possible pathways for broader public engagement. This workshop report identifies a set of nine Key Concepts. Each Concept is introduced with a concise overall statement, followed by a few important fundamentals. Connections to current and future technologies are included, providing relevance and context. The first Key Concept defines the field as a whole. Concepts 2-6 introduce ideas that are necessary for building an understanding of quantum information science and its applications. Concepts 7-9 provide short explanations of critical areas of study within QIS: quantum computing, quantum communication and quantum sensing. The Key Concepts are not intended to be an introductory guide to quantum information science, but rather provide a framework for future expansion and adaptation for students at different levels in computer science, mathematics, physics, and chemistry courses. As such, it is expected that educators and other community stakeholders may not yet have a working knowledge of content covered in the Key Concepts. -

Unit 1 Old Quantum Theory
UNIT 1 OLD QUANTUM THEORY Structure Introduction Objectives li;,:overy of Sub-atomic Particles Earlier Atom Models Light as clectromagnetic Wave Failures of Classical Physics Black Body Radiation '1 Heat Capacity Variation Photoelectric Effect Atomic Spectra Planck's Quantum Theory, Black Body ~diation. and Heat Capacity Variation Einstein's Theory of Photoelectric Effect Bohr Atom Model Calculation of Radius of Orbits Energy of an Electron in an Orbit Atomic Spectra and Bohr's Theory Critical Analysis of Bohr's Theory Refinements in the Atomic Spectra The61-y Summary Terminal Questions Answers 1.1 INTRODUCTION The ideas of classical mechanics developed by Galileo, Kepler and Newton, when applied to atomic and molecular systems were found to be inadequate. Need was felt for a theory to describe, correlate and predict the behaviour of the sub-atomic particles. The quantum theory, proposed by Max Planck and applied by Einstein and Bohr to explain different aspects of behaviour of matter, is an important milestone in the formulation of the modern concept of atom. In this unit, we will study how black body radiation, heat capacity variation, photoelectric effect and atomic spectra of hydrogen can be explained on the basis of theories proposed by Max Planck, Einstein and Bohr. They based their theories on the postulate that all interactions between matter and radiation occur in terms of definite packets of energy, known as quanta. Their ideas, when extended further, led to the evolution of wave mechanics, which shows the dual nature of matter -

Quantum Theory of the Hydrogen Atom
Quantum Theory of the Hydrogen Atom Chemistry 35 Fall 2000 Balmer and the Hydrogen Spectrum n 1885: Johann Balmer, a Swiss schoolteacher, empirically deduced a formula which predicted the wavelengths of emission for Hydrogen: l (in Å) = 3645.6 x n2 for n = 3, 4, 5, 6 n2 -4 •Predicts the wavelengths of the 4 visible emission lines from Hydrogen (which are called the Balmer Series) •Implies that there is some underlying order in the atom that results in this deceptively simple equation. 2 1 The Bohr Atom n 1913: Niels Bohr uses quantum theory to explain the origin of the line spectrum of hydrogen 1. The electron in a hydrogen atom can exist only in discrete orbits 2. The orbits are circular paths about the nucleus at varying radii 3. Each orbit corresponds to a particular energy 4. Orbit energies increase with increasing radii 5. The lowest energy orbit is called the ground state 6. After absorbing energy, the e- jumps to a higher energy orbit (an excited state) 7. When the e- drops down to a lower energy orbit, the energy lost can be given off as a quantum of light 8. The energy of the photon emitted is equal to the difference in energies of the two orbits involved 3 Mohr Bohr n Mathematically, Bohr equated the two forces acting on the orbiting electron: coulombic attraction = centrifugal accelleration 2 2 2 -(Z/4peo)(e /r ) = m(v /r) n Rearranging and making the wild assumption: mvr = n(h/2p) n e- angular momentum can only have certain quantified values in whole multiples of h/2p 4 2 Hydrogen Energy Levels n Based on this model, Bohr arrived at a simple equation to calculate the electron energy levels in hydrogen: 2 En = -RH(1/n ) for n = 1, 2, 3, 4, . -
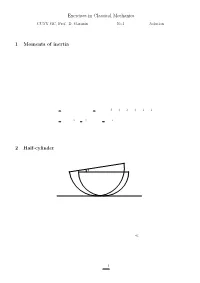
Exercises in Classical Mechanics 1 Moments of Inertia 2 Half-Cylinder
Exercises in Classical Mechanics CUNY GC, Prof. D. Garanin No.1 Solution ||||||||||||||||||||||||||||||||||||||||| 1 Moments of inertia (5 points) Calculate tensors of inertia with respect to the principal axes of the following bodies: a) Hollow sphere of mass M and radius R: b) Cone of the height h and radius of the base R; both with respect to the apex and to the center of mass. c) Body of a box shape with sides a; b; and c: Solution: a) By symmetry for the sphere I®¯ = I±®¯: (1) One can ¯nd I easily noticing that for the hollow sphere is 1 1 X ³ ´ I = (I + I + I ) = m y2 + z2 + z2 + x2 + x2 + y2 3 xx yy zz 3 i i i i i i i i 2 X 2 X 2 = m r2 = R2 m = MR2: (2) 3 i i 3 i 3 i i b) and c): Standard solutions 2 Half-cylinder ϕ (10 points) Consider a half-cylinder of mass M and radius R on a horizontal plane. a) Find the position of its center of mass (CM) and the moment of inertia with respect to CM. b) Write down the Lagrange function in terms of the angle ' (see Fig.) c) Find the frequency of cylinder's oscillations in the linear regime, ' ¿ 1. Solution: (a) First we ¯nd the distance a between the CM and the geometrical center of the cylinder. With σ being the density for the cross-sectional surface, so that ¼R2 M = σ ; (3) 2 1 one obtains Z Z 2 2σ R p σ R q a = dx x R2 ¡ x2 = dy R2 ¡ y M 0 M 0 ¯ 2 ³ ´3=2¯R σ 2 2 ¯ σ 2 3 4 = ¡ R ¡ y ¯ = R = R: (4) M 3 0 M 3 3¼ The moment of inertia of the half-cylinder with respect to the geometrical center is the same as that of the cylinder, 1 I0 = MR2: (5) 2 The moment of inertia with respect to the CM I can be found from the relation I0 = I + Ma2 (6) that yields " µ ¶ # " # 1 4 2 1 32 I = I0 ¡ Ma2 = MR2 ¡ = MR2 1 ¡ ' 0:3199MR2: (7) 2 3¼ 2 (3¼)2 (b) The Lagrange function L is given by L('; '_ ) = T ('; '_ ) ¡ U('): (8) The potential energy U of the half-cylinder is due to the elevation of its CM resulting from the deviation of ' from zero. -

Vibrational Quantum Number
Fundamentals in Biophotonics Quantum nature of atoms, molecules – matter Aleksandra Radenovic [email protected] EPFL – Ecole Polytechnique Federale de Lausanne Bioengineering Institute IBI 26. 03. 2018. Quantum numbers •The four quantum numbers-are discrete sets of integers or half- integers. –n: Principal quantum number-The first describes the electron shell, or energy level, of an atom –ℓ : Orbital angular momentum quantum number-as the angular quantum number or orbital quantum number) describes the subshell, and gives the magnitude of the orbital angular momentum through the relation Ll2 ( 1) –mℓ:Magnetic (azimuthal) quantum number (refers, to the direction of the angular momentum vector. The magnetic quantum number m does not affect the electron's energy, but it does affect the probability cloud)- magnetic quantum number determines the energy shift of an atomic orbital due to an external magnetic field-Zeeman effect -s spin- intrinsic angular momentum Spin "up" and "down" allows two electrons for each set of spatial quantum numbers. The restrictions for the quantum numbers: – n = 1, 2, 3, 4, . – ℓ = 0, 1, 2, 3, . , n − 1 – mℓ = − ℓ, − ℓ + 1, . , 0, 1, . , ℓ − 1, ℓ – –Equivalently: n > 0 The energy levels are: ℓ < n |m | ≤ ℓ ℓ E E 0 n n2 Stern-Gerlach experiment If the particles were classical spinning objects, one would expect the distribution of their spin angular momentum vectors to be random and continuous. Each particle would be deflected by a different amount, producing some density distribution on the detector screen. Instead, the particles passing through the Stern–Gerlach apparatus are deflected either up or down by a specific amount. -

The Quantum Mechanical Model of the Atom
The Quantum Mechanical Model of the Atom Quantum Numbers In order to describe the probable location of electrons, they are assigned four numbers called quantum numbers. The quantum numbers of an electron are kind of like the electron’s “address”. No two electrons can be described by the exact same four quantum numbers. This is called The Pauli Exclusion Principle. • Principle quantum number: The principle quantum number describes which orbit the electron is in and therefore how much energy the electron has. - it is symbolized by the letter n. - positive whole numbers are assigned (not including 0): n=1, n=2, n=3 , etc - the higher the number, the further the orbit from the nucleus - the higher the number, the more energy the electron has (this is sort of like Bohr’s energy levels) - the orbits (energy levels) are also called shells • Angular momentum (azimuthal) quantum number: The azimuthal quantum number describes the sublevels (subshells) that occur in each of the levels (shells) described above. - it is symbolized by the letter l - positive whole number values including 0 are assigned: l = 0, l = 1, l = 2, etc. - each number represents the shape of a subshell: l = 0, represents an s subshell l = 1, represents a p subshell l = 2, represents a d subshell l = 3, represents an f subshell - the higher the number, the more complex the shape of the subshell. The picture below shows the shape of the s and p subshells: (notice the electron clouds) • Magnetic quantum number: All of the subshells described above (except s) have more than one orientation. -

4 Nuclear Magnetic Resonance
Chapter 4, page 1 4 Nuclear Magnetic Resonance Pieter Zeeman observed in 1896 the splitting of optical spectral lines in the field of an electromagnet. Since then, the splitting of energy levels proportional to an external magnetic field has been called the "Zeeman effect". The "Zeeman resonance effect" causes magnetic resonances which are classified under radio frequency spectroscopy (rf spectroscopy). In these resonances, the transitions between two branches of a single energy level split in an external magnetic field are measured in the megahertz and gigahertz range. In 1944, Jevgeni Konstantinovitch Savoiski discovered electron paramagnetic resonance. Shortly thereafter in 1945, nuclear magnetic resonance was demonstrated almost simultaneously in Boston by Edward Mills Purcell and in Stanford by Felix Bloch. Nuclear magnetic resonance was sometimes called nuclear induction or paramagnetic nuclear resonance. It is generally abbreviated to NMR. So as not to scare prospective patients in medicine, reference to the "nuclear" character of NMR is dropped and the magnetic resonance based imaging systems (scanner) found in hospitals are simply referred to as "magnetic resonance imaging" (MRI). 4.1 The Nuclear Resonance Effect Many atomic nuclei have spin, characterized by the nuclear spin quantum number I. The absolute value of the spin angular momentum is L =+h II(1). (4.01) The component in the direction of an applied field is Lz = Iz h ≡ m h. (4.02) The external field is usually defined along the z-direction. The magnetic quantum number is symbolized by Iz or m and can have 2I +1 values: Iz ≡ m = −I, −I+1, ..., I−1, I. -

Analysis of Nonlinear Dynamics in a Classical Transmon Circuit
Analysis of Nonlinear Dynamics in a Classical Transmon Circuit Sasu Tuohino B. Sc. Thesis Department of Physical Sciences Theoretical Physics University of Oulu 2017 Contents 1 Introduction2 2 Classical network theory4 2.1 From electromagnetic fields to circuit elements.........4 2.2 Generalized flux and charge....................6 2.3 Node variables as degrees of freedom...............7 3 Hamiltonians for electric circuits8 3.1 LC Circuit and DC voltage source................8 3.2 Cooper-Pair Box.......................... 10 3.2.1 Josephson junction.................... 10 3.2.2 Dynamics of the Cooper-pair box............. 11 3.3 Transmon qubit.......................... 12 3.3.1 Cavity resonator...................... 12 3.3.2 Shunt capacitance CB .................. 12 3.3.3 Transmon Lagrangian................... 13 3.3.4 Matrix notation in the Legendre transformation..... 14 3.3.5 Hamiltonian of transmon................. 15 4 Classical dynamics of transmon qubit 16 4.1 Equations of motion for transmon................ 16 4.1.1 Relations with voltages.................. 17 4.1.2 Shunt resistances..................... 17 4.1.3 Linearized Josephson inductance............. 18 4.1.4 Relation with currents................... 18 4.2 Control and read-out signals................... 18 4.2.1 Transmission line model.................. 18 4.2.2 Equations of motion for coupled transmission line.... 20 4.3 Quantum notation......................... 22 5 Numerical solutions for equations of motion 23 5.1 Design parameters of the transmon................ 23 5.2 Resonance shift at nonlinear regime............... 24 6 Conclusions 27 1 Abstract The focus of this thesis is on classical dynamics of a transmon qubit. First we introduce the basic concepts of the classical circuit analysis and use this knowledge to derive the Lagrangians and Hamiltonians of an LC circuit, a Cooper-pair box, and ultimately we derive Hamiltonian for a transmon qubit. -

Path Integrals in Quantum Mechanics
Path Integrals in Quantum Mechanics Emma Wikberg Project work, 4p Department of Physics Stockholm University 23rd March 2006 Abstract The method of Path Integrals (PI’s) was developed by Richard Feynman in the 1940’s. It offers an alternate way to look at quantum mechanics (QM), which is equivalent to the Schrödinger formulation. As will be seen in this project work, many "elementary" problems are much more difficult to solve using path integrals than ordinary quantum mechanics. The benefits of path integrals tend to appear more clearly while using quantum field theory (QFT) and perturbation theory. However, one big advantage of Feynman’s formulation is a more intuitive way to interpret the basic equations than in ordinary quantum mechanics. Here we give a basic introduction to the path integral formulation, start- ing from the well known quantum mechanics as formulated by Schrödinger. We show that the two formulations are equivalent and discuss the quantum mechanical interpretations of the theory, as well as the classical limit. We also perform some explicit calculations by solving the free particle and the harmonic oscillator problems using path integrals. The energy eigenvalues of the harmonic oscillator is found by exploiting the connection between path integrals, statistical mechanics and imaginary time. Contents 1 Introduction and Outline 2 1.1 Introduction . 2 1.2 Outline . 2 2 Path Integrals from ordinary Quantum Mechanics 4 2.1 The Schrödinger equation and time evolution . 4 2.2 The propagator . 6 3 Equivalence to the Schrödinger Equation 8 3.1 From the Schrödinger equation to PI’s . 8 3.2 From PI’s to the Schrödinger equation . -

Quantum Mechanics
Quantum Mechanics Richard Fitzpatrick Professor of Physics The University of Texas at Austin Contents 1 Introduction 5 1.1 Intendedaudience................................ 5 1.2 MajorSources .................................. 5 1.3 AimofCourse .................................. 6 1.4 OutlineofCourse ................................ 6 2 Probability Theory 7 2.1 Introduction ................................... 7 2.2 WhatisProbability?.............................. 7 2.3 CombiningProbabilities. ... 7 2.4 Mean,Variance,andStandardDeviation . ..... 9 2.5 ContinuousProbabilityDistributions. ........ 11 3 Wave-Particle Duality 13 3.1 Introduction ................................... 13 3.2 Wavefunctions.................................. 13 3.3 PlaneWaves ................................... 14 3.4 RepresentationofWavesviaComplexFunctions . ....... 15 3.5 ClassicalLightWaves ............................. 18 3.6 PhotoelectricEffect ............................. 19 3.7 QuantumTheoryofLight. .. .. .. .. .. .. .. .. .. .. .. .. .. 21 3.8 ClassicalInterferenceofLightWaves . ...... 21 3.9 QuantumInterferenceofLight . 22 3.10 ClassicalParticles . .. .. .. .. .. .. .. .. .. .. .. .. .. .. 25 3.11 QuantumParticles............................... 25 3.12 WavePackets .................................. 26 2 QUANTUM MECHANICS 3.13 EvolutionofWavePackets . 29 3.14 Heisenberg’sUncertaintyPrinciple . ........ 32 3.15 Schr¨odinger’sEquation . 35 3.16 CollapseoftheWaveFunction . 36 4 Fundamentals of Quantum Mechanics 39 4.1 Introduction .................................. -

A Relativistic Electron in a Coulomb Potential
A Relativistic Electron in a Coulomb Potential Alfred Whitehead Physics 518, Fall 2009 The Problem Solve the Dirac Equation for an electron in a Coulomb potential. Identify the conserved quantum numbers. Specify the degeneracies. Compare with solutions of the Schrödinger equation including relativistic and spin corrections. Approach My approach follows that taken by Dirac in [1] closely. A few modifications taken from [2] and [3] are included, particularly in regards to the final quantum numbers chosen. The general strategy is to first find a set of transformations which turn the Hamiltonian for the system into a form that depends only on the radial variables r and pr. Once this form is found, I solve it to find the energy eigenvalues and then discuss the energy spectrum. The Radial Dirac Equation We begin with the electromagnetic Hamiltonian q H = p − cρ ~σ · ~p − A~ + ρ mc2 (1) 0 1 c 3 with 2 0 0 1 0 3 6 0 0 0 1 7 ρ1 = 6 7 (2) 4 1 0 0 0 5 0 1 0 0 2 1 0 0 0 3 6 0 1 0 0 7 ρ3 = 6 7 (3) 4 0 0 −1 0 5 0 0 0 −1 1 2 0 1 0 0 3 2 0 −i 0 0 3 2 1 0 0 0 3 6 1 0 0 0 7 6 i 0 0 0 7 6 0 −1 0 0 7 ~σ = 6 7 ; 6 7 ; 6 7 (4) 4 0 0 0 1 5 4 0 0 0 −i 5 4 0 0 1 0 5 0 0 1 0 0 0 i 0 0 0 0 −1 We note that, for the Coulomb potential, we can set (using cgs units): Ze2 p = −eΦ = − o r A~ = 0 This leads us to this form for the Hamiltonian: −Ze2 H = − − cρ ~σ · ~p + ρ mc2 (5) r 1 3 We need to get equation 5 into a form which depends not on ~p, but only on the radial variables r and pr.