Peters 1.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Drug Information Sheet("Kusuri-No-Shiori")
Drug Information Sheet("Kusuri-no-Shiori") Internal Published: 05/2017 The information on this sheet is based on approvals granted by the Japanese regulatory authority. Approval details may vary by country. Medicines have adverse reactions (risks) as well as efficacies (benefits). It is important to minimize adverse reactions and maximize efficacy. To obtain a better therapeutic response, patients should understand their medication and cooperate with the treatment. Brand name:PRANLUKAST TABLETS 112.5mg "CEO" Active ingredient:Pranlukast hydrate Dosage form:white to pale yellow tablet, diameter: 7.5 mm, thickness: 2.7 mm Print on wrapping:(face) プランルカスト 112.5mg「CEO」, CEO 131, プランルカスト 112.5mg (back) PRANLUKAST 112.5mg「CEO」, CEO 131, プランルカスト 112.5mg Effects of this medicine This medicine selectively binds to leukotriene receptor and inhibits its action. It consequently suppresses increase in airway contraction, vascular permeability, mucosal edema and hypersensitivity. It is usually used to treat bronchial asthma and allergic rhinitis. However, it cannot stop the attack of bronchial asthma already in progress but prevents the asthma attack. Before using this medicine, be sure to tell your doctor and pharmacist ・If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines. ・If you are pregnant or breastfeeding. ・If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.) Dosing schedule (How to take this medicine) ・Your dosing schedule prescribed by your doctor is(( to be written by a healthcare professional)) ・In general, for adults, take 2 tablets (225 mg of the active ingredient) at a time, twice a day after breakfast and dinner. -

Inhibitory Activity of Pranlukast and Montelukas Against Histamine
Showa Univ J Med Sci 21(2), 77~84, June 2009 Original Inhibitory Activity of Pranlukast and Montelukas Against Histamine Release and LTC4 Production from Human Basophils 1, 2 1 1 Satoshi HIBINO ), Ryoko ITO ), Taeru KITABAYASHI ), 1 2 Kazuo ITAHASHI ) and Toshio NAKADATE ) Abstract : Leukotriene receptor antagonists(LTRAs)are routinely used to treat bronchial asthma and are thought to act mostly by inhibiting leukotriene receptors. However, there is no preclinical or clinical evidence of the direct effect of LTRAs on histamine release from and leukotriene(LT)C4 produc- tion by basophils. We used anti-IgE antibody(Ab), FMLP, and C5a to induce histamine release, and anti-IgE Ab and FMLP to stimulate LTC 4 production. Basophils were exposed to different concentrations of pranlukast and montelu- kast, and then to anti-IgE Ab, FMLP, and C5a. Culture supernatant histamine and LTC 4 levels were measured by using a histamine ELISA kit and a LTC 4 EIA kit, respectively. Histamine release was expressed as a percentage of the total histamine content(%HR)induced by anti-IgE Ab, FMLP, or C5a. To evaluate the effects of pranlukast and montelukast on histamine release and LTC 4 production, we calculated the percent inhibition of histamine release and LTC 4 production, expressed as percent inhibition, at different concentrations of pranlukast and montelukast. Pranlukast significantly inhibited histamine release stimulated by FMLP and C5a, but had no effect on histamine release stimulated by anti-IgE Ab. By comparison, montelukast signicantly inhibited histamine release stimulated by FMLP, C5a, and anti-IgE Ab, in a concentration-dependent manner. Both pranlukast and montelukast signicantly inhibited LTC 4 production stimulated by anti-IgE Ab and FMLP. -

Drug Class Review Controller Medications for Asthma
Drug Class Review Controller Medications for Asthma Final Update 1 Report April 2011 The Agency for Healthcare Research and Quality has not yet seen or approved this report. The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Original Report: November 2008 Daniel E. Jonas, MD, MPH Roberta C. M. Wines, MPH Marcy DelMonte, PharmD, BCPS Halle R. Amick, MSPH Tania M. Wilkins, MS Brett D. Einerson, MPH Christine L. Schuler, MD Blake A. Wynia, MPH Betsy Bryant Shilliday, Pharm.D., CDE, CPP Produced by RTI-UNC Evidence-based Practice Center Cecil G. Sheps Center for Health Services Research University of North Carolina at Chapel Hill 725 Martin Luther King Jr. Blvd, CB# 7590 Chapel Hill, NC 27599-7590 Tim Carey, M.D., M.P.H., Director Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Copyright © 2011 by Oregon Health & Science University Portland, Oregon 97239. All rights reserved. Final Update 1 Report Drug Effectiveness Review Project The medical literature relating to this topic is scanned periodically. (See http://www.ohsu.edu/xd/research/centers-institutes/evidence-based-policy- center/derp/documents/methods.cfm for description of scanning process). Prior versions of this report can be accessed at the DERP website. -

Understanding Interparticle Interactions in Dry Powder Inhalation: Glass Beads As an Innovative Model Carrier System
UNDERSTANDING INTERPARTICLE INTERACTIONS IN DRY POWDER INHALATION: GLASS BEADS AS AN INNOVATIVE MODEL CARRIER SYSTEM DOCTORAL THESIS SUMBITTED IN FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR IN NATURAL SCIENCES AT KIEL UNIVERSITY, GERMANY by Niklas Ludwig Renner Kiel 2017 Referee: Prof. Dr. Regina Scherließ Co-Referee: Prof. Dr. Thomas Kunze Date of Exam: 13.10.2017 Accepted for publication: 13.10.2017 Prof. Dr. Natascha Oppelt (Dean) Published research articles contributing to the present thesis: Renner, N.; Steckel, H.; Urbanetz, N.A.; Scherließ, R. Tailoring the surface topography of a model carrier to alter dry powder inhaler performance Dalby, R.N. (Ed.), RDD Europe 2017 2 (2017), 195-200 Renner, N; Steckel, H.; Urbanetz, N.A.; Scherließ, R. Nano- and microstructured model carrier surfaces to alter dry powder inhaler performance International Journal of Pharmaceutics 518 (2017), 20-28 Conference contributions: Renner, N.; Steckel, H.; Urbanetz, N.A.; Scherließ, R. Tailoring the surface topography of a model carrier to alter dry powder inhaler per- formance Respiratory Drug Delivery, Nice, France (2017) Renner, N.; Steckel, H.; Urbanetz, N.A.; Scherließ, R. A deeper insight into the impact of chemical surface properties on inhalation perfor- mance Drug Delivery to the Lungs 27, Edinburgh, Scotland (2016) Renner, N.; Scherließ, R.; Steckel, H. Glass beads as model carriers in dry powder inhalers: the influence of chemical sur- face properties on inhalation performance International Congress on Particle Technology, Nurnberg, Germany (2016) Renner, N.; Scherließ, R.; Steckel, H. Investigating the influence of carrier surface roughness on drug delivery in DPIs 10th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Tech- nology, Glasgow, Scotland (2016) Renner, N.; Kutelova, Z.; Scherließ, R.; Steckel, H. -

The Role of 5-Lipoxygenase in Pathophysiology and Management of Neuropathic Pain
REVIEW ARTICLE THE ROLE OF 5-LIPOXYGENASE IN PATHOPHYSIOLOGY AND MANAGEMENT OF NEUROPATHIC PAIN Pascanus Lamsihar PT∗, Faldi Yaputra∗∗, Jimmy FA Barus4 and I Putu Eka Widyadharma∗∗,1 ∗Department of Neurology, Provincial Mental Hospital, West Borneo, Indonesia., ∗∗Department of Neurology, Faculty of Medicine, Udayana University-Sanglah General Hospital, Bali, Indonesia., 4Department of Neurology, Faculty of Medicine, Atma Jaya Catholic University, Jakarta-Indonesia. ABSTRACT Neuropathic pain (NP) is a pain caused by lesions in the nervous system. Several causes of NP are traumatic, metabolic disorders, ischemia, toxins, infections, immune-related, and hereditary. The pathophysiology of NP is very complicated and unknown entirely. Therefore the treatment of NP is still unsatisfactory. Recent studies believed the critical role of primary inflammatory mediators in the pathophysiology of NP especially leukotrienes (LTs). The 5- lipoxygenase enzyme (5-LOX) is an enzyme that plays a role in the metabolism of arachidonic acid into LTs. Leukotrienes (LTs) are the essential inflammatory mediators in the pathophysiology of NP. Leukotriene B4 (LTB4) can cause chemotaxis on neutrophils, lowering nociceptors threshold and may contribute to NP. Several studies believed the administration of 5-LOX inhibitors or LTs receptor antagonists could be useful in the management of NP. The purpose of this review is to summarize the involvement of 5-LOX enzyme as an essential role in the pathophysiology and management of NP. KEYWORDS 5-lipoxygenase, leukotrienes, -

The Role of Pharmacogenomics in Improving the Management of Asthma
The Role of Pharmacogenomics in Improving the Management of Asthma The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Kazani S, ME Wechsler, E. Israel. 2010. TThe Role of Pharmacogenomics in Improving the Management of Asthma. Journal of Allergy and Clinical Immunology 125, no. 2: 295–302. Published Version 10.1016/j.jaci.2009.12.014 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:42659246 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Open Access Policy Articles, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#OAP NIH Public Access Author Manuscript J Allergy Clin Immunol. Author manuscript; available in PMC 2011 February 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: J Allergy Clin Immunol. 2010 February ; 125(2): 295±302. doi:10.1016/j.jaci.2009.12.014. THE ROLE OF PHARMACOGENOMICS IN IMPROVING THE MANAGEMENT OF ASTHMA Shamsah Kazani, M.D. [Instructor in Medicine] Harvard Medical School Brigham and Women's Hospital Boston, Massachusetts Michael E. Wechsler, M.D. [Assistant Professor of Medicine] Harvard Medical School Brigham and Women's Hospital Boston, Massachusetts Elliot Israel, M.D. [Associate Professor of Medicine] Harvard Medical School Brigham and Women's Hospital Boston, Massachusetts Abstract There is a large amount of interindividual variability in both therapeutic and adverse responses to asthma therapies. Genetic variability may account for 50–60% of this variability. -
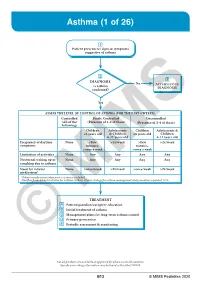
Asthma (1 of 26)
Asthma (1 of 26) 1 Patient presents w/ signs & symptoms suggestive of asthma 2 3 DIAGNOSIS No ALTERNATIVE Is asthma DIAGNOSIS confi rmed? Yes ASSESS THE LEVEL OF CONTROL OF ASTHMA FOR THE PAST 4 WEEKS Controlled Partly Controlled Uncontrolled (All of the (Presence of 1-2 of these) (Presence of 3-4 of these) following) Children Adolescents Children Adolescents & ≤5 years old & Children ≤5 years old Children 6-11 years old 6-11 years old Frequency of daytime None >Few >2x/week >Few >2x/week symptoms minutes, minutes, >once a week >once a week Limitation of activities None Any Any Any Any Nocturnal waking up or None Any Any Any Any coughing due to asthma Need for reliever None >once/week >2x/week >once/week >2x/week medication* *Reliever medications taken prior to exercise excluded. Modified from: Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention: Updated 2020. TREATMENT A Patient/guardian/caregiver education B Initial treatment of asthma C Management plans for long-term asthma control D Primary prevention E © Periodic assessmentMIMS & monitoring Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B13 © MIMS Pediatrics 2020 Asthma (2 of 26) 1 ASTHMA • A heterogeneous disease w/ chronic infl ammatory disorder of the airways • e most common chronic disease in pediatric age groups that causes signifi cant morbidity • Characterized by history of respiratory symptoms eg wheeze, shortness of breath, chest tightness & cough -

Chapter 5 Drug Promotion, Clinical Trials, and Conflicts of Interest
Chapter 5 Drug Promotion, Clinical Trials, and Conflicts of Interest "Gifts buy you time with a doc, time that might change his mind. .Money is the big resource. The pads and pens are great for access, but the dinners and what costs money. CDs, handheld computers, everything given in the name of research this is what's thrown at docs to get them to change their minds." A Former Detailer Drug industry lobbies do not appreciate people who squeal, the outstanding instance is the documented case by Stanley Adams in his book Roche Versus Adams.1 Stanley Adams was an executive who did what he felt was right by alerting the European Commission to cartelisation and anti-competitive practices by Swiss-based pharmaceutical giant Hoffmann-La Roche. The Commission fined Hoffmann for abuse of its dominant position in the bulk vitamin market but during antitrust proceedings disclosed information that enabled Hoffmann to identify Adams, who was consequently arrested and convicted for unauthorised disclosure under Swiss law. Adams was hounded by Swiss law, arrested while crossing the borders, and thereafter things went horribly wrong for him and his family including the 'suicide' of his wife. Adams successfully sought damages from the Commission, which was held by the European Court to have failed its obligation "not to disclose information of the kind covered by the obligation of professional secrecy, in particular information about undertakings, their business relations or their cost components." In 1999, Roche was fined US $500 million in the US for a repeat of its offence. A more recent instance is that of cardiologist Dr. -

2020 GINA Pocket Guide for Asthma Management and Prevention
POCKET GUIDE FOR ASTHMA MANAGEMENT AND PREVENTION (for Adults and Children Older than 5 Years) DISTRIBUTE OR COPY NOT DO MATERIAL- COPYRIGHTED A Pocket Guide for Health Professionals Updated 2020 BASED ON THE GLOBAL STRATEGY FOR ASTHMA MANAGEMENT AND PREVENTION © 2020 Global Initiative for Asthma GLOBAL INITIATIVE FOR ASTHMA ASTHMA MANAGEMENT AND PREVENTION DISTRIBUTE for adults and children older ORthan 5 years COPY NOT A POCKET GUIDE FOR HEALTHDO PROFESSIONALS MATERIAL-Updated 2020 GINA Science Committee Chair: Helen Reddel, MBBS PhD COPYRIGHTED GINA Board of Directors Chair: Louis-Philippe Boulet, MD GINA Dissemination and Implementation Committee Chair: Mark Levy, MD (to Sept 2019); Alvaro Cruz, MD (from Sept 2019) GINA Assembly The GINA Assembly includes members from many countries, listed on the GINA website www.ginasthma.org. GINA Executive Director Rebecca Decker, BS, MSJ Names of members of the GINA Committees are listed on page 48. 1 LIST OF ABBREVIATIONS BDP Beclometasone dipropionate COPD Chronic obstructive pulmonary disease CXR Chest X-ray DPI Dry powder inhaler FeNO Fraction of exhaled nitric oxide FEV1 Forced expiratory volume in 1 second FVC Forced vital capacity GERD Gastroesophageal reflux disease HDM House dust mite ICS Inhaled corticosteroids ICS-LABA Combination ICS and LABA Ig Immunoglobulin IL Interleukin DISTRIBUTE IV Intravenous OR LABA Long-acting beta2-agonist COPY LAMA Long-acting muscarinic antagonist NOT LTRA Leukotriene receptor antagonistDO n.a. Not applicable NSAID Nonsteroidal anti-inflammatory drug MATERIAL- O2 Oxygen OCS Oral corticosteroids PEF Peak expiratory flow pMDI PressurizedCOPYRIGHTED metered dose inhaler SABA Short-acting beta2-agonist SC Subcutaneous SLIT Sublingual immunotherapy 2 TABLE OF CONTENTS List of abbreviations ........................................................................................ -

Inflammation, Cancer and Oxidative Lipoxygenase Activity Are Intimately Linked
Cancers 2014, 6, 1500-1521; doi:10.3390/cancers6031500 OPEN ACCESS cancers ISSN 2072-6694 www.mdpi.com/journal/cancers Review Inflammation, Cancer and Oxidative Lipoxygenase Activity are Intimately Linked Rosalina Wisastra and Frank J. Dekker * Pharmaceutical Gene Modulation, Groningen Research Institute of Pharmacy, University of Groningen, Antonius Deusinglaan 1, 9713 AV Groningen, The Netherlands; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +31-5-3638030; Fax: +31-5-3637953. Received: 16 April 2014; in revised form: 27 June 2014 / Accepted: 2 July 2014 / Published: 17 July 2014 Abstract: Cancer and inflammation are intimately linked due to specific oxidative processes in the tumor microenvironment. Lipoxygenases are a versatile class of oxidative enzymes involved in arachidonic acid metabolism. An increasing number of arachidonic acid metabolites is being discovered and apart from their classically recognized pro-inflammatory effects, anti-inflammatory effects are also being described in recent years. Interestingly, these lipid mediators are involved in activation of pro-inflammatory signal transduction pathways such as the nuclear factor κB (NF-κB) pathway, which illustrates the intimate link between lipid signaling and transcription factor activation. The identification of the role of arachidonic acid metabolites in several inflammatory diseases led to a significant drug discovery effort around arachidonic acid metabolizing enzymes. However, to date success in this area has been limited. This might be attributed to the lack of selectivity of the developed inhibitors and to a lack of detailed understanding of the functional roles of arachidonic acid metabolites in inflammatory responses and cancer. -

Antileukotriene Agents in Asthma: the Dart That Kills the Elephant?
Antileukotriene agents in asthma: The dart that kills the elephant? Education Paolo M. Renzi, MD Éducation Abstract From the Research Centre and the Pulmonary Unit, THE PERSISTENCE OF AIRWAY INFLAMMATION is believed to cause the mechanical Centre hospitalier de changes and symptoms of asthma. After decades of research, a new class of med- l’Université de Montréal ication has emerged that focuses on leukotrienes, mediators of inflammation. These Hospitals, Université de substances are potent inducers of bronchoconstriction, increased vascular perme- Montréal, and the Meakins ability and mucus production, and they potentiate the influx of inflammatory cells Christie Laboratories, McGill in the airways of patients with asthma. In this article the author reviews the devel- University, Montreal, Que. opment, mechanism of action, and clinical and toxic effects of the leukotriene syn- thesis inhibitors and receptor antagonists that are entering the North American market. These agents can decrease airway response to antigen, airway hyperre- This article has been peer reviewed. sponsiveness and exercise-induced asthma. They are also effective inhibitors of ASA-induced symptoms. Although few published studies are available, the an- CMAJ 1999;160:217-23 tileukotrienes seem almost as effective in the management of chronic asthma as ß See related article page 209 low-dose inhaled corticosteroids, and their use permits a decrease in the frequency of use or dose of corticosteroids. Further evaluation and clinical experience will determine the position of targeted inhibition of the leukotriene pathway in the treatment of asthma. Résumé ON CROIT QUE LA PERSISTANCE DE L’INFLAMMATION des voies respiratoires provoque les changements mécaniques et les symptômes de l’asthme. -

ANNUAL INSTITUTIONAL PROFILE September 1, 2011
ANNUAL INSTITUTIONAL PROFILE September 1, 2011 WE TEACH. WE DISCOVER. WE HEAL. WE CARE. Office of Institutional Research University Office of Academic Affairs UMDNJ–ANNUAL INSTITUTIONAL PROFILE, SEPTEMBER 1, 2011 INTRODUCTION The University of Medicine and Dentistry of New Jersey (UMDNJ) is New Jersey’s public research university dedicated to excellence in the health sciences. As a statewide health, education and research resource, UMDNJ benefits every region of New Jersey. Since it was created in 1970, the University has expanded to better serve our state with eight schools on five geographically distinct campuses across the state. We conduct research and offer graduate degrees, certificates, and undergraduate degrees in multiple fields of study including: medical, dental, allied health, nursing, public health and biomedical sciences disciplines. The University also owns a teaching and safety-net hospital that is accessible to all; New Jersey’s only NCI-designated comprehensive cancer center; a statewide network of behavioral healthcare providers; and has scores of partnerships and affiliations with leading healthcare facilities, institutions of higher learning, and agencies throughout New Jersey. UMDNJ is a truly unique and wonderful statewide asset, and as our credo states, “We embrace our responsibility to the people of New Jersey.” In this spirit, we proudly present this year’s report to the Commission on Higher Education and to the people of New Jersey. William F. Owen, Jr., MD President UMDNJ-ANNUAL INSTITUTIONAL PROFILE, SEPTEMBER