Planck's Radiation Law
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blackbody Radiation: (Vibrational Energies of Atoms in Solid Produce BB Radiation)
Independent study in physics The Thermodynamic Interaction of Light with Matter Mirna Alhanash Project in Physics Uppsala University Contents Abstract ................................................................................................................................................................................ 3 Introduction ......................................................................................................................................................................... 3 Blackbody Radiation: (vibrational energies of atoms in solid produce BB radiation) .................................... 4 Stefan-Boltzmann .............................................................................................................................................................. 6 Wien displacement law..................................................................................................................................................... 7 Photoelectric effect ......................................................................................................................................................... 12 Frequency dependence/Atom model & electron excitation .................................................................................. 12 Why we see colours ....................................................................................................................................................... 14 Optical properties of materials: .................................................................................................................................. -

Statistical Physics Problem Sets 5–8: Statistical Mechanics
Statistical Physics xford hysics Second year physics course A. A. Schekochihin and A. Boothroyd (with thanks to S. J. Blundell) Problem Sets 5{8: Statistical Mechanics Hilary Term 2014 Some Useful Constants −23 −1 Boltzmann's constant kB 1:3807 × 10 JK −27 Proton rest mass mp 1:6726 × 10 kg 23 −1 Avogadro's number NA 6:022 × 10 mol Standard molar volume 22:414 × 10−3 m3 mol−1 Molar gas constant R 8:315 J mol−1 K−1 1 pascal (Pa) 1 N m−2 1 standard atmosphere 1:0132 × 105 Pa (N m−2) 1 bar (= 1000 mbar) 105 N m−2 Stefan{Boltzmann constant σ 5:67 × 10−8 Wm−2K−4 2 PROBLEM SET 5: Foundations of Statistical Mechanics If you want to try your hand at some practical calculations first, start with the Ideal Gas questions Maximum Entropy Inference 5.1 Factorials. a) Use your calculator to work out ln 15! Compare your answer with the simple version of Stirling's formula (ln N! ≈ N ln N − N). How big must N be for the simple version of Stirling's formula to be correct to within 2%? b∗) Derive Stirling's formula (you can look this up in a book). If you figure out this derivation, you will know how to calculate the next term in the approximation (after N ln N − N) and therefore how to estimate the precision of ln N! ≈ N ln N − N for any given N without calculating the factorials on a calculator. Check the result of (a) using this method. -

Influence of Boundary Conditions on Statistical Properties of Ideal Bose
PHYSICAL REVIEW E, VOLUME 65, 036129 Influence of boundary conditions on statistical properties of ideal Bose-Einstein condensates Martin Holthaus* Fachbereich Physik, Carl von Ossietzky Universita¨t, D-26111 Oldenburg, Germany Kishore T. Kapale and Marlan O. Scully Max-Planck-Institut fu¨r Quantenoptik, D-85748 Garching, Germany and Department of Physics and Institute for Quantum Studies, Texas A&M University, College Station, Texas 77843 ͑Received 23 October 2001; published 27 February 2002͒ We investigate the probability distribution that governs the number of ground-state particles in a partially condensed ideal Bose gas confined to a cubic volume within the canonical ensemble. Imposing either periodic or Dirichlet boundary conditions, we derive asymptotic expressions for all its cumulants. Whereas the conden- sation temperature becomes independent of the boundary conditions in the large-system limit, as implied by Weyl’s theorem, the fluctuation of the number of condensate particles and all higher cumulants remain sensi- tive to the boundary conditions even in that limit. The implications of these findings for weakly interacting Bose gases are briefly discussed. DOI: 10.1103/PhysRevE.65.036129 PACS number͑s͒: 05.30.Ch, 05.30.Jp, 03.75.Fi ϭ When London, in 1938, wrote his now-famous papers on with 1/(kBT), where kB denotes Boltzmann’s constant. Bose-Einstein condensation of an ideal gas ͓1,2͔, he simply Note that the product runs over the excited states only, ex- considered a free system of N noninteracting Bose particles cluding the ground state ϭ0; the frequencies of the indi- without an external trapping potential, imposing periodic vidual oscillators being given by the excited-states energies boundary conditions on a cubic volume V. -

Section 22-3: Energy, Momentum and Radiation Pressure
Answer to Essential Question 22.2: (a) To find the wavelength, we can combine the equation with the fact that the speed of light in air is 3.00 " 108 m/s. Thus, a frequency of 1 " 1018 Hz corresponds to a wavelength of 3 " 10-10 m, while a frequency of 90.9 MHz corresponds to a wavelength of 3.30 m. (b) Using Equation 22.2, with c = 3.00 " 108 m/s, gives an amplitude of . 22-3 Energy, Momentum and Radiation Pressure All waves carry energy, and electromagnetic waves are no exception. We often characterize the energy carried by a wave in terms of its intensity, which is the power per unit area. At a particular point in space that the wave is moving past, the intensity varies as the electric and magnetic fields at the point oscillate. It is generally most useful to focus on the average intensity, which is given by: . (Eq. 22.3: The average intensity in an EM wave) Note that Equations 22.2 and 22.3 can be combined, so the average intensity can be calculated using only the amplitude of the electric field or only the amplitude of the magnetic field. Momentum and radiation pressure As we will discuss later in the book, there is no mass associated with light, or with any EM wave. Despite this, an electromagnetic wave carries momentum. The momentum of an EM wave is the energy carried by the wave divided by the speed of light. If an EM wave is absorbed by an object, or it reflects from an object, the wave will transfer momentum to the object. -

151545957.Pdf
Universit´ede Montr´eal Towards a Philosophical Reconstruction of the Dialogue between Modern Physics and Advaita Ved¯anta: An Inquiry into the Concepts of ¯ak¯a´sa, Vacuum and Reality par Jonathan Duquette Facult´ede th´eologie et de sciences des religions Th`ese pr´esent´ee `ala Facult´edes ´etudes sup´erieures en vue de l’obtention du grade de Philosophiae Doctor (Ph.D.) en sciences des religions Septembre 2010 c Jonathan Duquette, 2010 Universit´ede Montr´eal Facult´edes ´etudes sup´erieures et postdoctorales Cette th`ese intitul´ee: Towards A Philosophical Reconstruction of the Dialogue between Modern Physics and Advaita Ved¯anta: An Inquiry into the Concepts of ¯ak¯a´sa, Vacuum and Reality pr´esent´ee par: Jonathan Duquette a ´et´e´evalu´ee par un jury compos´edes personnes suivantes: Patrice Brodeur, pr´esident-rapporteur Trichur S. Rukmani, directrice de recherche Normand Mousseau, codirecteur de recherche Solange Lefebvre, membre du jury Varadaraja Raman, examinateur externe Karine Bates, repr´esentante du doyen de la FESP ii Abstract Toward the end of the 19th century, the Hindu monk and reformer Swami Vivekananda claimed that modern science was inevitably converging towards Advaita Ved¯anta, an important philosophico-religious system in Hinduism. In the decades that followed, in the midst of the revolution occasioned by the emergence of Einstein’s relativity and quantum physics, a growing number of authors claimed to discover striking “par- allels” between Advaita Ved¯anta and modern physics. Such claims of convergence have continued to the present day, especially in relation to quantum physics. In this dissertation, an attempt is made to critically examine such claims by engaging a de- tailed comparative analysis of two concepts: ¯ak¯a´sa in Advaita Ved¯anta and vacuum in quantum physics. -

Kelvin's Clouds
Kelvin’s clouds Oliver Passon∗ July 1, 2021 Abstract In 1900 Lord Kelvin identified two problems for 19th century physics, two“clouds” as he puts it: the relative motion of the ether with respect to massive objects, and Maxwell-Boltzmann’s theorem on the equipartition of energy. These issues were eventually solved by the theory of special relativity and by quantum mechanics. In modern quotations, the content of Kelvin’s lecture is almost always distorted and misrepresented. For example, it is commonly claimed that Kelvin was concerned about the UV- catastrophe of the Rayleigh-Jeans law, while his lecture actually makes no reference at all to blackbody radiation. I rectify these mistakes and explore reasons for their occurrence. 1 Introduction In 1900, Lord Kelvin, born as William Thomson, delivered a lecture in which he identified two problems for physics at the turn of the 20th century: the question of the existence of ether and the equipartition theorem. The solution for these two clouds was eventually achieved by the theory of special relativity and by quantum mechanics [1]. Understandably, these prophetic remarks are often quoted in popular science books or in the introductory sections of textbooks on modern physics. A typical example is found in the textbook by Tipler and Llewellyn [2]: [...] as there were already vexing cracks in the foundation of what we now refer to as classical physics. Two of these were described by Lord Kelvin, in his famous Baltimore Lectures in 1900, as the “two clouds” on the horizon of twentieth-century physics: the failure of arXiv:2106.16033v1 [physics.hist-ph] 30 Jun 2021 theory to account for the radiation spectrum emitted by a blackbody and the inexplicable results of the Michelson-Morley experiment. -

Note-A-Rific: Blackbody Radiation
Note-A-Rific: Blackbody Radiation What is Blackbody How to Calculate Peak Failure of Classical Radiation Wavelength Physics What is Blackbody Radiation? As thermal energy is added to an object to heat it up, the object will emit radiation at various wavelengths. • All objects emit radiation at an intensity equal to its temperature (in degrees Kelvin, see Glossary) to the fourth power. 4 Intensity = T o At room temperatures, we are not aware of objects emitting radiation because it’s at such a low intensity. o At higher temperatures there is enough infrared radiation that we can feel it. o At still higher temperatures (about 1000K) we can actually see the object glowing red o At temperatures above 2000K, objects glow yellowish or white hot. No real object will absorb all radiation falling on it, nor will it re-emit all of the energy it absorbs. • Instead physicists came up with a theoretical model called a Blackbody. • A blackbody absorbs all radiation falling on it, and then releases all that energy as radiation in the form of EM waves. • As the temperature of a blackbody increases, it will emit more and more intense radiation. • At the same time, as the temperature increases, most of the radiation is released at higher and higher frequencies (lower and lower wavelengths). • The frequency at which the emitted radiation is at the highest intensity is called the peak frequency or (more typically) the peak wavelength. • This explains why an object at room temperature does not emit much radiation, and what radiation it does emit is at higher wavelengths, like infrared. -
Properties of Electromagnetic Waves Any Electromagnetic Wave Must Satisfy Four Basic Conditions: 1
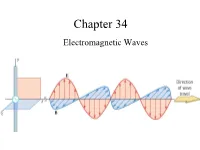
Chapter 34 Electromagnetic Waves The Goal of the Entire Course Maxwell’s Equations: Maxwell’s Equations James Clerk Maxwell •1831 – 1879 •Scottish theoretical physicist •Developed the electromagnetic theory of light •His successful interpretation of the electromagnetic field resulted in the field equations that bear his name. •Also developed and explained – Kinetic theory of gases – Nature of Saturn’s rings – Color vision Start at 12:50 https://www.learner.org/vod/vod_window.html?pid=604 Correcting Ampere’s Law Two surfaces S1 and S2 near the plate of a capacitor are bounded by the same path P. Ampere’s Law states that But it is zero on S2 since there is no conduction current through it. This is a contradiction. Maxwell fixed it by introducing the displacement current: Fig. 34-1, p. 984 Maxwell hypothesized that a changing electric field creates an induced magnetic field. Induced Fields . An increasing solenoid current causes an increasing magnetic field, which induces a circular electric field. An increasing capacitor charge causes an increasing electric field, which induces a circular magnetic field. Slide 34-50 Displacement Current d d(EA)d(q / ε) 1 dq E 0 dt dt dt ε0 dt dq d ε E dt0 dt The displacement current is equal to the conduction current!!! Bsd μ I μ ε I o o o d Maxwell’s Equations The First Unified Field Theory In his unified theory of electromagnetism, Maxwell showed that electromagnetic waves are a natural consequence of the fundamental laws expressed in these four equations: q EABAdd 0 εo dd Edd s BE B s μ I μ ε dto o o dt QuickCheck 34.4 The electric field is increasing. -

The Two-Dimensional Bose Gas in Box Potentials Laura Corman
The two-dimensional Bose Gas in box potentials Laura Corman To cite this version: Laura Corman. The two-dimensional Bose Gas in box potentials. Physics [physics]. Université Paris sciences et lettres, 2016. English. NNT : 2016PSLEE014. tel-01449982 HAL Id: tel-01449982 https://tel.archives-ouvertes.fr/tel-01449982 Submitted on 30 Jan 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE DE DOCTORAT de l’Université de recherche Paris Sciences Lettres – PSL Research University préparée à l’École normale supérieure The Two-Dimensional Bose École doctorale n°564 Gas in Box Potentials Spécialité: Physique Soutenue le 02.06.2016 Composition du Jury : par Laura Corman M Tilman Esslinger ETH Zürich Rapporteur Mme Hélène Perrin Université Paris XIII Rapporteur M Zoran Hadzibabic Cambridge University Membre du Jury M Gilles Montambaux Université Paris XI Membre du Jury M Jean Dalibard Collège de France Directeur de thèse M Jérôme Beugnon Université Paris VI Membre invité ABSTRACT Degenerate atomic gases are a versatile tool to study many-body physics. They offer the possibility to explore low-dimension physics, which strongly differs from the three dimensional (3D) case due to the enhanced role of fluctuations. -

Blackbody Radiation and the Quantization of Energy
Blackbody Radiation and the Quantization of Energy Energy quantization, the noncontinuous nature of energy,was discovered from observing radiant energy from blackb o dies. A blackbody absorbs electromagnetic EM energy eciently. An ideal blackb o dy absorbs all electromagnetic energy that b ears up on it. Ecient absorb ers of energy also are ecient emitters or else they would heat up without limit. Energy radiated from blackb o dies was studied b ecause the characteristics of the radiation are indep endent of the material constitution of the blackb o dy. Therefore, conclusions reached by the study of energy radiated from blackb o dies do not require quali cations related to the material studied. If you heat up a go o d absorb er emitter of EM energy, it will radiate energy with total p ower p er unit area prop ortional to the fourth p ower of temp erature in degrees Kelvin. This is the Stefan - Boltzmann law: 4 R = T ; 8 2 4 where the Stefan constant =5:6703 10 W/m K . This lawwas rst prop osed by Josef Stefan in 1879 and studied theoretically by Boltzmann a few years later, so it is named after b oth of them. The EM energy emitted from a blackb o dy actually dep ends strongly on the wavelength, , of the energy. Thus, radiantpower is a function of wavelength, R , and total p ower p er unit area is simply an integral over all wavelengths: Z R = R d: 1 The function R is called the blackbody spectrum. Recall that ! 2 c = f = ! =2f k = k The blackb o dy sp ectrum is di erent for di erent temp eratures, but has the same general shap e as the solid lines in Figure 1 show. -

Condensation of Bosons with Several Degrees of Freedom Condensación De Bosones Con Varios Grados De Libertad
Condensation of bosons with several degrees of freedom Condensación de bosones con varios grados de libertad Trabajo presentado por Rafael Delgado López1 para optar al título de Máster en Física Fundamental bajo la dirección del Dr. Pedro Bargueño de Retes2 y del Prof. Fernando Sols Lucia3 Universidad Complutense de Madrid Junio de 2013 Calificación obtenida: 10 (MH) 1 [email protected], Dep. Física Teórica I, Universidad Complutense de Madrid 2 [email protected], Dep. Física de Materiales, Universidad Complutense de Madrid 3 [email protected], Dep. Física de Materiales, Universidad Complutense de Madrid Abstract The condensation of the spinless ideal charged Bose gas in the presence of a magnetic field is revisited as a first step to tackle the more complex case of a molecular condensate, where several degrees of freedom have to be taken into account. In the charged bose gas, the conventional approach is extended to include the macroscopic occupation of excited kinetic states lying in the lowest Landau level, which plays an essential role in the case of large magnetic fields. In that limit, signatures of two diffuse phase transitions (crossovers) appear in the specific heat. In particular, at temperatures lower than the cyclotron frequency, the system behaves as an effectively one-dimensional free boson system, with the specific heat equal to (1/2) NkB and a gradual condensation at lower temperatures. In the molecular case, which is currently in progress, we have studied the condensation of rotational levels in a two–dimensional trap within the Bogoliubov approximation, showing that multi–step condensation also occurs. -

Solid State Physics
Solid State Physics Chetan Nayak Physics 140a Franz 1354; M, W 11:00-12:15 Office Hour: TBA; Knudsen 6-130J Section: MS 7608; F 11:00-11:50 TA: Sumanta Tewari University of California, Los Angeles September 2000 Contents 1 What is Condensed Matter Physics? 1 1.1 Length, time, energy scales . 1 1.2 Microscopic Equations vs. States of Matter, Phase Transitions, Critical Points ................................... 2 1.3 Broken Symmetries . 3 1.4 Experimental probes: X-ray scattering, neutron scattering, NMR, ther- modynamic, transport . 3 1.5 The Solid State: metals, insulators, magnets, superconductors . 4 1.6 Other phases: liquid crystals, quasicrystals, polymers, glasses . 5 2 Review of Quantum Mechanics 7 2.1 States and Operators . 7 2.2 Density and Current . 10 2.3 δ-function scatterer . 11 2.4 Particle in a Box . 11 2.5 Harmonic Oscillator . 12 2.6 Double Well . 13 2.7 Spin . 15 2.8 Many-Particle Hilbert Spaces: Bosons, Fermions . 15 3 Review of Statistical Mechanics 18 ii 3.1 Microcanonical, Canonical, Grand Canonical Ensembles . 18 3.2 Bose-Einstein and Planck Distributions . 21 3.2.1 Bose-Einstein Statistics . 21 3.2.2 The Planck Distribution . 22 3.3 Fermi-Dirac Distribution . 23 3.4 Thermodynamics of the Free Fermion Gas . 24 3.5 Ising Model, Mean Field Theory, Phases . 27 4 Broken Translational Invariance in the Solid State 30 4.1 Simple Energetics of Solids . 30 4.2 Phonons: Linear Chain . 31 4.3 Quantum Mechanics of a Linear Chain . 31 4.3.1 Statistical Mechnics of a Linear Chain .