Starting Pages.Pmd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download PDF Version
MarLIN Marine Information Network Information on the species and habitats around the coasts and sea of the British Isles Lagoon cockle (Cerastoderma glaucum) MarLIN – Marine Life Information Network Biology and Sensitivity Key Information Review Nicola White 2002-07-15 A report from: The Marine Life Information Network, Marine Biological Association of the United Kingdom. Please note. This MarESA report is a dated version of the online review. Please refer to the website for the most up-to-date version [https://www.marlin.ac.uk/species/detail/1315]. All terms and the MarESA methodology are outlined on the website (https://www.marlin.ac.uk) This review can be cited as: White, N. 2002. Cerastoderma glaucum Lagoon cockle. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. DOI https://dx.doi.org/10.17031/marlinsp.1315.1 The information (TEXT ONLY) provided by the Marine Life Information Network (MarLIN) is licensed under a Creative Commons Attribution-Non-Commercial-Share Alike 2.0 UK: England & Wales License. Note that images and other media featured on this page are each governed by their own terms and conditions and they may or may not be available for reuse. Permissions beyond the scope of this license are available here. Based on a work at www.marlin.ac.uk (page left blank) Date: 2002-07-15 Lagoon cockle (Cerastoderma glaucum) - Marine Life Information Network See online review for distribution map Three Cerastoderma glaucum with siphons extended. Distribution data supplied by the Ocean Photographer: Dennis R. -

Review of Selected Species Subject to Long- Standing Import Suspensions
UNEP-WCMC technical report Review of selected species subject to long- standing import suspensions Part II: Asia and Oceania (Version edited for public release) Review of selected species subject to long-standing import suspensions. Part II: Asia and Oceania Prepared for The European Commission, Directorate General Environment, Directorate E - Global & Regional Challenges, LIFE ENV.E.2. – Global Sustainability, Trade & Multilateral Agreements, Brussels, Belgium Prepared February 2016 Copyright European Commission 2016 Citation UNEP-WCMC. 2016. Review of selected species subject to long-standing import suspensions. Part II: Asia and Oceania. UNEP-WCMC, Cambridge. The UNEP World Conservation Monitoring Centre (UNEP-WCMC) is the specialist biodiversity assessment of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organization. The Centre has been in operation for over 30 years, combining scientific research with policy advice and the development of decision tools. We are able to provide objective, scientifically rigorous products and services to help decision- makers recognize the value of biodiversity and apply this knowledge to all that they do. To do this, we collate and verify data on biodiversity and ecosystem services that we analyze and interpret in comprehensive assessments, making the results available in appropriate forms for national and international level decision-makers and businesses. To ensure that our work is both sustainable and equitable we seek to build the capacity of partners -

ENCORE: the Eаect of Nutrient Enrichment on Coral Reefs
Marine Pollution Bulletin Vol. 42, No. 2, pp. 91±120, 2001 Ó 2001 Published by Elsevier Science Ltd. Printed in Great Britain PII: S0025-326X$00)00181-8 0025-326X/01 $ - see front matter ENCORE: The Eect of Nutrient Enrichment on Coral Reefs. Synthesis of Results and Conclusions K. KOOP *,1, D. BOOTHà, A. BROADBENT§,2, J. BRODIE , D. BUCHERàà, D. CAPONE ,3, J. COLL§§,4, W. DENNISON , M. ERDMANNààà, P. HARRISONàà, O. HOEGH-GULDBERG ,5, P. HUTCHINGS§§§, G. B. JONES§, A. W. D. LARKUM , J. O'NEIL ,5, A. STEVEN ,6, E. TENTORI§§, S. WARDàà,5, J. WILLIAMSON ,7 and D. YELLOWLEESàààà School of Biological Sciences, The University of Sydney, Sydney NSW 2006, Australia àDepartment Environmental Sciences, University of Technology, Sydney NSW 2065 Australia §Department of Chemistry, James Cook University, Townsville, Qld 4810, Australia Great Barrier Reef Marine Park Authority, P.O. Box 1379, Townsville, Qld 4810, Australia ààCentre for Coastal Management, Southern Cross University, P.O. Box 157, Lismore NSW 2480, Australia §§Department of Biology, Central Queensland University, Rockhampton, Qld 4702, Australia Department of Botany, University of Queensland, Brisbane, Qld 4072, Australia àààP.O. Box 1020, Manado, Sulawesi, Indonesia §§§The Australian Museum, 6, College Street, Sydney, NSW 2010, Australia Chesapeake Biological Laboratory, University of Maryland, Box 38, Solomons, MA 20688-0038, USA ààààBiochemistry and Molecular Biology, James Cook University, Townsville, Qld 4811 Australia Coral reef degradation resulting from nutrient enrichment assessed a variety of factors focusing on nutrient dynamics of coastal waters is of increasing global concern. Although and biotic responses. A controlled and replicated experi- eects of nutrients on coral reef organisms have been ment was conducted over two years using twelve small demonstrated in the laboratory,there is little direct evi- patch reefs ponded at low tide by a coral rim. -

Spatial Variability in Recruitment of an Infaunal Bivalve
Spatial Variability in Recruitment of an Infaunal Bivalve: Experimental Effects of Predator Exclusion on the Softshell Clam (Mya arenaria L.) along Three Tidal Estuaries in Southern Maine, USA Author(s): Brian F. Beal, Chad R. Coffin, Sara F. Randall, Clint A. Goodenow Jr., Kyle E. Pepperman, Bennett W. Ellis, Cody B. Jourdet and George C. Protopopescu Source: Journal of Shellfish Research, 37(1):1-27. Published By: National Shellfisheries Association https://doi.org/10.2983/035.037.0101 URL: http://www.bioone.org/doi/full/10.2983/035.037.0101 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Journal of Shellfish Research, Vol. 37, No. 1, 1–27, 2018. SPATIAL VARIABILITY IN RECRUITMENT OF AN INFAUNAL BIVALVE: EXPERIMENTAL EFFECTS OF PREDATOR EXCLUSION ON THE SOFTSHELL CLAM (MYA ARENARIA L.) ALONG THREE TIDAL ESTUARIES IN SOUTHERN MAINE, USA 1,2 3 2 3 BRIAN F. -

Tridacna Maxima (Reding), and Hippopus Hippopus (Linnaeus)!
Pacific Science (1976), Vol. 30, No.3, p. 219-233 Printed in Great Britain Early Life History of the Giant Clams Tridacna crocea Lamarck, Tridacna maxima (Reding), and Hippopus hippopus (Linnaeus)! STEPHEN C. JAMESON2 ABST RACT: Giant clams may be stimulated to spawn by the addition of macer ated gonads to the water.Individuals of Tridacna maxima collected at Anae Island, Guam, spawned from N ovember to March. On Palau, Hippopus hippopus spawned in June and Tridacna crocea, in July. Tridacna crocea, T. maxima, and H. hippopus displayed a stereotype d develop ment pattern in morphogenesis and rate of development . Fertilized eggs of T. crocea, T . maxima, and H. hippopus had mean diameters of 93.1, 104.5, and 130.0 psx», respectively . The day-2 straight-hinge veligers of T . crocea, T . maxima, and H. hippopus had mean shell lengths of 155.0, 168.0, and 174.4 pm, respec tively. Settlement occurred 12, 11, and 9 days after fertili zation at a mean shell length of 168.0, 195.0, and 202.0 pm for T . crocea,T . max ima, and H. bippopss, respectively. Metamorphosis was basically complete about 1 day after settlement. Juveniles of T . crocea, T. max ima, and H. hippopus first acqui re zooxanthellae after 19, 21, and 25 days, respectively. Growth rates increase sharply after the acquisition of zoo xanthellae. Juvenile shells show first signs of becoming opaque after 47 days for T . maxima and after 50 days for H. hippopus. G rANT CLAMS are protandric fun ctional herma formation, and initial organogenesis. The larval phrodites (Wada 1942, 1952). -

Outer Ards Modiolus Modiolus Report
R Assessment of Outer Ards Modiolus modiolus biogenic reefs against Special Area of Conservation (SAC) criteria JULY 2016 A report from the Fisheries and Aquatic Ecosystems Branch, Agri-food and Biosciences Institute to The Department of Agriculture, Environment and Rural Affairs (Northern Ireland) Document version control: Version Issue date Modifier Note Issued to and date 1.0 31/03/2016 AFBI-AC First draft for review DAERA & MS: 31/03/2016 1.1 19/05/2016 AFBI-AC Second draft for review DAERA & MS: 19/05/2016 1.2 19/07/2016 AFBI-AC Final draft for sign off following MS: 19/07/2016 receipt of comments 22/06/2016 1.3 19/07/2016 AFBI-AC Final version DAERA: 20/07/2016 Further information Dr. Annika Clements Seabed Habitat Mapping Project Leader Fisheries & Aquatic Ecosystems Branch Newforge Lane Belfast BT9 5PX Tel: +44(0)2890255153 Email: [email protected] DAERA Client Officer Joe Breen DAERA, Marine Conservation and Reporting Team Marine & Fisheries Division Portrush Coastal Zone 8 Bath Road PORTRUSH BT56 8AP Tel: +44(0)2870823600 (ext31) Email: [email protected] The GIS project “Outer_Ards_Modiolus_2016.mxd” should be available for use in conjunction with this report. Recommended citation: AFBI, 2016. Special Area of Conservation Designation Assessment of Outer Ards Modiolus modiolus Biogenic Reef. Report to the Department of Agriculture, Environment and Rural Affairs, Northern Ireland. Acknowledgements The author wishes to thank Adele Boyd and Matthew Service (AFBI) for data provision, James McArdle (AFBI), Katie Lilley (Ulster University placement student with AFBI) and Clara Alvarez Alonso (DAERA) and the master and crew of the R.V. -

Genetic Variability in the Indonesian Giant Clam (Tridacna Crocea and Tridacna Maxima) Populations: Implication for Mariculture and Restocking Program
Genetic Variability in the Indonesian Giant Clam (Tridacna crocea and Tridacna maxima) Populations: Implication for Mariculture and Restocking Program Agus Nuryanto1, Dedy Duryadi2, Dedi Soedharma3, Dietmar Blohm4 1 Faculty of Biology, Jenderal Soedirman University, Purwokerto 2 Department of Biology, Faculty of Mathematics and Life Sciences, Bogor Agriculture University 3 Faculty of Fisheries and Marine Sciences, Bogor Agriculture University 4 Department of Biotechnology and Molecular Genetics, FB2-UFT, University of Bremen Abstract Tridacna crocea and T. maxima are relatively abundant in the Indonesian coral reef. These two species are, however, under high presure due to exploitation for food, industry, and aquarium trade. It is, therefore, necessary to understand their biology, such as genetic variability within and between populations, before utilizing them for strain improvement and restocking, prior to the extinction of the populations of T. crocea and T. maxima. Here we amplified a length of 456 bp of the mitochondrial DNA cytochrome c oxidase I gene from Tridacna crocea and of 484 bp from T. maxima to asses the genetic variability within and between populations of both species. The results showed that both species have high genetic diversity and polymorphism within each local population. This provides a sufficient basis for selection of improved strain of T. crocea and T. maxima for mariculture. However, if the genetic variation led to genetic differentiation among populations due to the result of evolutionary adaptation, mixing genetically different populations may result in the break up of co-adaptation gene complexes. This might result in the loss of the physiological capacities of the parental populations. Key words: genetic variability, cytochrome c oxidase I gene, Tridacna crocea, Tridacna maxima Introduction The family of tridacnidae, also known as giant clam, is conspicuous bivalve that inhabits coral reef across the Indo-Pacific region (Lucas, 1988). -

TREATISE ONLINE Number 48
TREATISE ONLINE Number 48 Part N, Revised, Volume 1, Chapter 31: Illustrated Glossary of the Bivalvia Joseph G. Carter, Peter J. Harries, Nikolaus Malchus, André F. Sartori, Laurie C. Anderson, Rüdiger Bieler, Arthur E. Bogan, Eugene V. Coan, John C. W. Cope, Simon M. Cragg, José R. García-March, Jørgen Hylleberg, Patricia Kelley, Karl Kleemann, Jiří Kříž, Christopher McRoberts, Paula M. Mikkelsen, John Pojeta, Jr., Peter W. Skelton, Ilya Tëmkin, Thomas Yancey, and Alexandra Zieritz 2012 Lawrence, Kansas, USA ISSN 2153-4012 (online) paleo.ku.edu/treatiseonline PART N, REVISED, VOLUME 1, CHAPTER 31: ILLUSTRATED GLOSSARY OF THE BIVALVIA JOSEPH G. CARTER,1 PETER J. HARRIES,2 NIKOLAUS MALCHUS,3 ANDRÉ F. SARTORI,4 LAURIE C. ANDERSON,5 RÜDIGER BIELER,6 ARTHUR E. BOGAN,7 EUGENE V. COAN,8 JOHN C. W. COPE,9 SIMON M. CRAgg,10 JOSÉ R. GARCÍA-MARCH,11 JØRGEN HYLLEBERG,12 PATRICIA KELLEY,13 KARL KLEEMAnn,14 JIřÍ KřÍž,15 CHRISTOPHER MCROBERTS,16 PAULA M. MIKKELSEN,17 JOHN POJETA, JR.,18 PETER W. SKELTON,19 ILYA TËMKIN,20 THOMAS YAncEY,21 and ALEXANDRA ZIERITZ22 [1University of North Carolina, Chapel Hill, USA, [email protected]; 2University of South Florida, Tampa, USA, [email protected], [email protected]; 3Institut Català de Paleontologia (ICP), Catalunya, Spain, [email protected], [email protected]; 4Field Museum of Natural History, Chicago, USA, [email protected]; 5South Dakota School of Mines and Technology, Rapid City, [email protected]; 6Field Museum of Natural History, Chicago, USA, [email protected]; 7North -

HETA ROUSI: Zoobenthos As Indicators of Marine Habitats in the Northern Baltic
Heta Rousi Zoobenthos as indicators of marine Heta Rousi | habitats in the northern Baltic Sea of marine as indicators habitats in the northernZoobenthos Baltic Sea Heta Rousi This thesis describes how physical and chemical environmental variables impact zoobenthic species distribution in the northern Baltic Sea and how dis- Zoobenthos as indicators of marine tinct zoobenthic species indicate different marine benthic habitats. The thesis inspects the effects of habitats in the northern Baltic Sea depth, sediment type, temperature, salinity, oxy- gen, nutrients as well as topographical and geo- logical factors on zoobenthos on small and large temporal and spatial scales. | 2020 ISBN 978-952-12-3944-1 Heta Rousi Född 1979 Studier och examina Magister vid Helsingfors Universitet 2006 Licentiat vid Åbo Akademi 2013 Doktorsexamen vid Åbo Akademi 2020 Institutionen för miljö- och marinbiologi, Åbo Akademi ZOOBENTHOS AS INDICATORS OF MARINE HABITATS IN THE NORTHERN BALTIC SEA HETA ROUSI Environmental and Marine Biology Faculty of Science and Engineering Åbo Akademi University Finland, 2020 SUPERVISED BY PRE-EXAMINED BY Professor Erik Bonsdorff Research Professor (Supervisor & Examiner) Markku Viitasalo Åbo Akademi University Finnish Environment Institute Faculty of Science and Engineering Sustainable Use of the Marine Areas Environmental and Marine Biology Latokartanonkaari 11 Artillerigatan 6 00790 Helsinki 20520 Åbo Finland Finland Professor Emeritus Ilppo Vuorinen CO-SUPERVISOR University of Turku Adjunct Professor Faculty of Science and Engineering Samuli Korpinen Itäinen Pitkäkatu 4 Finnish Environment Institute 20520 Turku Marine Management Finland Latokartanonkaari 11 00790 Helsinki FACULTY OPPONENT Finland Associate Professor Urszula Janas SUPERVISING AT THE University of Gdansk LICENCIATE PHASE Institute of Oceanography Assistant Professor Al. -
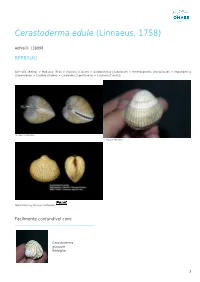
Cerastoderma Edule (Linnaeus, 1758)
Cerastoderma edule (Linnaeus, 1758) AphiaID: 138998 BERBIGÃO Animalia (Reino) > Mollusca (Filo) > Bivalvia (Classe) > Autobranchia (Subclasse) > Heteroconchia (Infraclasse) > Imparidentia (Superordem) > Cardiida (Ordem) > Cardioidea (Superfamilia) > Cardiidae (Familia) © Vasco Ferreira © Vasco Ferreira Natural History Museum Rotterdam Facilmente confundível com: Cerastoderma glaucum Berbigão 1 Estatuto de Conservação Sinónimos Cardium belgicum De Malzine, 1867 Cardium crenulatum Lamarck, 1819 Cardium edule Linnaeus, 1758 Cardium edule burchanae Girscher, 1938 Cardium edule var. batesoni Bucquoy, Dautzenberg & Dollfus, 1892 Cardium edule var. loppensi Mars, 1951 Cardium edule var. maculata Dautzenberg, 1890 Cardium edule var. major Bucquoy, Dautzenberg & Dollfus, 1892 Cardium edule var. mareotica Pallary, 1912 Cardium edule var. regularis Pallary, 1900 Cardium edule var. sibenicensis Brusina, 1870 Cardium mercatorium Coen, 1915 Cardium nunninkae Lucas, 1984 Cardium obtritum Locard, 1886 Cardium quadrarium Reeve, 1845 Cardium vulgare da Costa, 1778 Cardium vulgatum Tryon, 1872 Cerastoderma edule var. sinicola Lacourt, 1974 Cerastoderma nunninkae Lucas, 1984 Referências basis of record Gofas, S.; Le Renard, J.; Bouchet, P. (2001). Mollusca. in: Costello, M.J. et al. (eds), European Register of Marine Species: a check-list of the marine species in Europe and a bibliography of guides to their identification. Patrimoines Naturels. 50: 180-213. [details] additional source Poorten, J.J. ter, 2005. Outline of a systematic index – Recent Cardiidae (Lamarck, 1809). VISAYA net. (Updated 2009 for WoRMS), available online at http://www.conchology.be/en/shelltopics/visaya-net/date.php?year=2005 [details] 2 ecology source Coscia, I., P.E. Robins, J.S. Porter, S.K. Malham & J.E. Ironside. (2013). Modelled larval dispersal and measured gene flow: seascape genetics of the common cockle Cerastoderma edule in the southern Irish Sea. -

The Ecological Significance of Giant Clams in Coral Reef Ecosystems
Biological Conservation 181 (2015) 111–123 Contents lists available at ScienceDirect Biological Conservation journal homepage: www.elsevier.com/locate/biocon Review The ecological significance of giant clams in coral reef ecosystems ⇑ Mei Lin Neo a,b, William Eckman a, Kareen Vicentuan a,b, Serena L.-M. Teo b, Peter A. Todd a, a Experimental Marine Ecology Laboratory, Department of Biological Sciences, National University of Singapore, 14 Science Drive 4, Singapore 117543, Singapore b Tropical Marine Science Institute, National University of Singapore, 18 Kent Ridge Road, Singapore 119227, Singapore article info abstract Article history: Giant clams (Hippopus and Tridacna species) are thought to play various ecological roles in coral reef Received 14 May 2014 ecosystems, but most of these have not previously been quantified. Using data from the literature and Received in revised form 29 October 2014 our own studies we elucidate the ecological functions of giant clams. We show how their tissues are food Accepted 2 November 2014 for a wide array of predators and scavengers, while their discharges of live zooxanthellae, faeces, and Available online 5 December 2014 gametes are eaten by opportunistic feeders. The shells of giant clams provide substrate for colonization by epibionts, while commensal and ectoparasitic organisms live within their mantle cavities. Giant clams Keywords: increase the topographic heterogeneity of the reef, act as reservoirs of zooxanthellae (Symbiodinium spp.), Carbonate budgets and also potentially counteract eutrophication via water filtering. Finally, dense populations of giant Conservation Epibiota clams produce large quantities of calcium carbonate shell material that are eventually incorporated into Eutrophication the reef framework. Unfortunately, giant clams are under great pressure from overfishing and extirpa- Giant clams tions are likely to be detrimental to coral reefs. -

Taxonomy of Indonesian Giant Clams (Cardiidae, Tridacninae)
BIODIVERSITAS ISSN: 1412-033X Volume 13, Number 3, July 2012 E-ISSN: 2085-4722 Pages: 118-123 DOI: 10.13057/biodiv/d130303 Taxonomy of Indonesian giant clams (Cardiidae, Tridacninae) UDHI EKO HERNAWAN♥ Biotic Conservation Area of Tual Sea, Research Center for Oceanography, Indonesian Institute of Sciences. Jl. Merdeka, Katdek Tual, Southeast Maluku 97611. Tel. +92-916-23839, Fax. +62-916-23873, ♥email: [email protected] Manuscript received: 20 December 2010. Revision accepted: 20 June 2011. ABSTRACT Hernawan E. 2012. Taxonomy of Indonesian giant clams (Cardiidae, Tridacninae). Biodiversitas 13: 118-123. A taxonomic study was conducted on the giant clam’s specimens deposited in Museum Zoologicum Bogoriense (MZB), Cibinong Indonesia. Taxonomic overviews of the examined specimens are given with diagnostic characters, remarks, habitat and distribution. Discussion is focused on specific characters distinguishing each species. From seven species known to distribute in Indonesian waters, there are six species, Tridacna squamosa Lamarck, 1819; T. gigas Linnaeus, 1758; T. derasa Roding, 1798; T. crocea Lamarck, 1819; T. maxima Roding,1798; and Hippopus hippopus Linnaeus, 1758. This study suggests the need for collecting specimen of H. porcellanus Rosewater, 1982. Important characters to distinguish species among Tridacninae are interlocking teeth on byssal orifice, life habits, presence of scales and inhalant siphon tentacles. Key words: Tridacninae, taxonomy, Museum Zoologicum Bogoriense INTRODUCTION family (Tridacnidae) or revised to be subfamily Tridacninae, included in family Cardiidae. Recently, based Giant clams, the largest bivalve in the world, occur on sperm ultrastructure and molecular phylogenetic studies, naturally in association with coral reefs throughout the the clams are belonging to family Cardiidae, subfamily tropical and subtropical waters of the Indo-Pacific region.