Serotonergic Modulation of Visceral Sensation: Lower Gut M Camilleri
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FDA Warns About an Increased Risk of Serious Pancreatitis with Irritable Bowel Drug Viberzi (Eluxadoline) in Patients Without a Gallbladder
FDA warns about an increased risk of serious pancreatitis with irritable bowel drug Viberzi (eluxadoline) in patients without a gallbladder Safety Announcement [03-15-2017] The U.S. Food and Drug Administration (FDA) is warning that Viberzi (eluxadoline), a medicine used to treat irritable bowel syndrome with diarrhea (IBS-D), should not be used in patients who do not have a gallbladder. An FDA review found these patients have an increased risk of developing serious pancreatitis that could result in hospitalization or death. Pancreatitis may be caused by spasm of a certain digestive system muscle in the small intestine. As a result, we are working with the Viberzi manufacturer, Allergan, to address these safety concerns. Patients should talk to your health care professional about how to control your symptoms of irritable bowel syndrome with diarrhea (IBS-D), particularly if you do not have a gallbladder. The gallbladder is an organ that stores bile, one of the body’s digestive juices that helps in the digestion of fat. Stop taking Viberzi right away and get emergency medical care if you develop new or worsening stomach-area or abdomen pain, or pain in the upper right side of your stomach-area or abdomen that may move to your back or shoulder. This pain may occur with nausea and vomiting. These may be symptoms of pancreatitis, an inflammation of the pancreas, an organ important in digestion; or spasm of the sphincter of Oddi, a muscular valve in the small intestine that controls the flow of digestive juices to the gut. Health care professionals should not prescribe Viberzi in patients who do not have a gallbladder and should consider alternative treatment options in these patients. -

Therapeutic Class Overview Irritable Bowel Syndrome Agents
Therapeutic Class Overview Irritable Bowel Syndrome Agents Therapeutic Class Overview/Summary: This review will focus on agents used for the treatment of Irritable Bowel Syndrome (IBS).1-5 IBS is a gastrointestinal syndrome characterized primarily by non-specific chronic abdominal pain, usually described as a cramp-like sensation, and abnormal bowel habits, either constipation or diarrhea, in which there is no organic cause. Other common gastrointestinal symptoms may include gastroesophageal reflux, dysphagia, early satiety, intermittent dyspepsia and nausea. Patients may also experience a wide range of non-gastrointestinal symptoms. Some notable examples include sexual dysfunction, dysmenorrhea, dyspareunia, increased urinary frequency/urgency and fibromyalgia-like symptoms.6 IBS is defined by one of four subtypes. IBS with constipation (IBS-C) is the presence of hard or lumpy stools with ≥25% of bowel movements and loose or watery stools with <25% of bowel movements. When IBS is associated with diarrhea (IBS-D) loose or watery stools are present with ≥25% of bowel movements and hard or lumpy stools are present with <25% of bowel movements. Mixed IBS (IBS-M) is defined as the presence of hard or lumpy stools with ≥25% and loose or water stools with ≥25% of bowel movements. Final subtype, or unsubtyped, is all other cases of IBS that do not fall into the other classes. Pharmacological therapy for IBS depends on subtype.7 While several over-the-counter or off-label prescription agents are used for the treatment of IBS, there are currently only two agents approved by the Food and Drug Administration (FDA) for the treatment of IBS-C and three agents approved by the FDA for IBS-D. -

Prophylactic Antiemetic Therapy with Ondansetron, Granisetron and Metoclopramide in Patients Undergoing Laparoscopic Cholecystectomy Under GA
JK SCIENCE ORIGINAL ARTICLE Prophylactic Antiemetic Therapy with Ondansetron, Granisetron and Metoclopramide in Patients Undergoing Laparoscopic Cholecystectomy Under GA Vishal Gupta, Renu Wakhloo, Anjali Mehta, Satya Dev Gupta Abstract The aim of the present study was to compare the antiemetic effect of intravenous Granisetron, Ondansetron & Metoclopramide in a randomized blinded study for prophylaxis of post operative nausea and vomiting (PONV) in patients undergoing laparoscopic cholecystectomy under general anaesthesia. 60 patients (ASA I & II) undergoing laparoscopic cholecystectomy under general anaesthesia were randomly allocated into three equal groups (n=20). Emetic episodes in first 24 hours were recorded and compared in different study groups. Results were analyzed. Minimal emetic episodes were observed in early post-operative period (1-12hrs) in patients who had received intravenous granisetron in comparison to ondansetron and metoclopramide. However, after 12 hours emesis free periods were statistically insignificant between group A and B while patients in group C had no antiemetic effect. Keywords Post Operative Nausea and Vomiting (PONV), Granisetron, Ondensetron, Metoclopramide Introduction The most common and distressing symptoms, which vascular anastomoses and increased intracranial follow anaesthesia and surgery, are pain and emesis. The pressure(4). The anaesthetic consequences are aspiration syndrome of nausea, retching and vomiting is known as pneumonitis and discomfort in recovery. For institutions 'sickness' and each part of it can be distinguished as a there is increased financial burden because of increased separate entity (1). PONV (post operative nausea and nursing care, delayed discharge from Phase I and II vomiting) has been characterized as big 'little problem(2) recovery units and unexpected admissions. Hence, and has been a common complication for both in patients prophylactic antiemetic therapy is needed for all these and out patients undergoing virtually all types of surgical patients. -

Comprehensive Pgx Report for 1 / 31 Examples of Different Levels of Evidence for Pgx Snps
Comprehensive PGx report for PERSONAL DETAILS Advanced Diagnostics Laboratory LLC CLIA:31D2149403 Phone: Fax: PATIENT DOB Address: 1030 North Kings Highway Suite 304 Cherry Hill, NJ 08034 GENDER FEMALE Website: http://advanceddiagnosticslaboratory.com/ SPECIMEN TYPE Oral Fluid LABORATORY INFORMATION ORDERING PHYSICIAN ACCESSION NUMBER 100344 FACILITY COLLECTION DATE 08/10/2020 RECEIVED DATE 08/14/2020 REPORT GENERATED 09/08/2020 LABORATORY DIRECTOR Dr. Jeanine Chiaffarano Current Patient Medication Clonidine (Catapres, Kapvay) The personalized pharmacogenomics profile of this patient reveals intermediate CYP2D6-mediated metabolism, extensive CYP1A2-mediated metabolism, and extensive CYP3A5-mediated metabolism. For further details, please find supporting evidence in this report or on websites such as www.pharmgkb.org or www.fda.gov. Losartan (Cozaar) The personalized pharmacogenomics profile of this patient reveals extensive CYP2C9-mediated metabolism, extensive CYP3A4-mediated metabolism, and extensive CYP3A5-mediated metabolism. For further details, please find supporting evidence in this report or on websites such as www.pharmgkb.org or www.fda.gov. Diltiazem (Cardizem, Tiazac) The personalized pharmacogenomics profile of this patient reveals extensive CYP3A4-mediated metabolism, intermediate CYP2C19-mediated metabolism, and extensive CYP3A5- mediated metabolism. For further details, please find supporting evidence in this report or on websites such as www.pharmgkb.org or www.fda.gov. Labetalol (Normodyne, Trandate) The personalized pharmacogenomics profile of this patient reveals intermediate CYP2D6-mediated metabolism, and intermediate CYP2C19-mediated metabolism. For further details, please find supporting evidence in this report or on websites such as www.pharmgkb.org or www.fda.gov. Mycophenolate mofetil (Myfortic, CellCept) The personalized pharmacogenomics profile of this patient reveals extensive CYP3A4-mediated metabolism, extensive CYP3A5-mediated metabolism, and extensive CYP2C8-mediated metabolism. -

THE HARD TRUTH ABOUT PROKINETIC MEDICATION USE in PETS Introduction Pathophysiology/Etiology to That Observed in Dogs
VETTALK Volume 15, Number 04 American College of Veterinary Pharmacists THE HARD TRUTH ABOUT PROKINETIC MEDICATION USE IN PETS Introduction Pathophysiology/Etiology to that observed in dogs. It can be The moving topic of this Vet Talk As with most diseases in the veteri- due to a trichobezoar, dehydration, newsletter will be prokinetic medica- nary world, the etiology and patho- obesity, old age, diabetes, immobility, tions. The availability of information physiology of constipation are varied pain from trauma to the low back, on the many prokinetic agents is var- depending on the species being dis- bladder infection, or an anal sac infec- ied at best so an overall consensus of cussed, where in their gastrointestinal tion. In cases that are more chronic, prokinetic medications will be as- tract the problem is occurring, and underlying disease such as colitis or sessed in this article, hopefully giving any accompanying comorbid condi- Irritable Bowel Syndrome (IBS) may better insight to practitioners about tions. be the culprit. On the other hand, the which agents to use in their patients. cause may be idiopathic which is Canines: In man’s best friend, consti- frustrating for both veterinarian and Prevalence pation has many origins. A dog’s patient since this form is most diffi- Chronic constipation and gastroin- digestive tract itself is complex but cult to treat. testinal stasis are highly debilitating ultimately the mass movements and conditions that not only affect human haustral contractions from the large Equines: Despite their large size, patients but our four legged patients intestine (colon), propel feces into the horses have incredibly delicate diges- as well! Though this condition is rectum stimulating the internal anal tive systems. -

The Pharmacology of Prokinetic Agents and Their Role in the Treatment of Gastrointestinal Disorders
The Pharmacology of ProkineticAgents IJGE Issue 4 Vol 1 2003 Review Article The Pharmacology of Prokinetic Agents and Their Role in the Treatment of Gastrointestinal Disorders George Y. Wu, M.D, Ph.D. INTRODUCTION Metoclopramide Normal peristalsis of the gut requires complex, coordinated neural and motor activity. Pharmacologic Category : Gastrointestinal Abnormalities can occur at a number of different Agent. Prokinetic levels, and can be caused by numerous etiologies. This review summarizes current as well as new Symptomatic treatment of diabetic gastric agents that show promise in the treatment of stasis gastrointestinal motility disorders. For these Gastroesophageal reflux e conditions, the most common medications used in s Facilitation of intubation of the small the US are erythromycin, metoclopramide, and U intestine neostigmine (in acute intestinal pseudo- Prevention and/or treatment of nausea and obstruction). A new prokinetic agent, tegaserod, vomiting associated with chemotherapy, has been recently approved, while other serotonin radiation therapy, or post-surgery (1) agonist agents (prucalopride, YM-31636, SK-951, n ML 10302) are currently undergoing clinical o Blocks dopamine receptors in chemoreceptor i t studies. Other prokinetics, such as domperidone, c trigger zone of the CNS (2) A are not yet approved in the US, although are used in f Enhances the response to acetylcholine of o other countries. tissue in the upper GI tract, causing enhanced m s i n motility and accelerated gastric emptying a DELAYED GASTRIC EMPTYING OR h without stimulating gastric, biliary, or c G A S T R O E S O P H A G E A L R E F L U X e pancreatic secretions. -

Antidepressants for Functional Gastrointestinal Disorders
Antidepressants for the treatment of Functional Gastrointestinal Disorders Commonly IBS, constipation, diarrhea, functional abdominal pain and esophageal hypersensitivity Document adapted from literature available from the UNC Center for Functional GI & Motility Disorders What are Functional Gastrointestinal Disorders (FGIDs)? There are many different FGIDs (over 20), but among them, IBS is the most common. FGIDs are characterized by abnormal changes in the movement of muscles throughout the intestines (motility abnormality), an increase in the sensations produced by digestive tract activity (visceral hypersensitivity), and brain-gut dysfunction, especially in the brain’s ability to regulate painful signals from the GI tract. People with IBS have an increased awareness and interpretation of these activities as being abnormal. Motility Abnormality Visceral Hypersensitivity Brain-Gut Dysfunction Instead of normal muscular People with IBS, and other When nerve impulses from the gut reach activity (motility) during digestion, FGIDs, may experience an the brain, they may be experienced as people with IBS may experience increased sensitivity in the more severe or less severe based on the painful spasms and cramping. If nerves of the GI tract. This can regulatory activity of the brain-gut axis. motility is too fast it may produce happen after a GI infection or Signals of pain or discomfort travel from diarrhea and if it is too slow it operation which causes injury the intestines back to the brain. The may result in constipation. Motility to the nerves. This produces a brain usually has the ability to “turn abnormalities may be associated lower pain threshold for normal down” the pain by sending signals that with: cramping, belching, digestive sensations, leading to block nerve impulses produced in the GI urgency, and abdominal pain and discomfort. -

5-HT3 Receptor Antagonists in Neurologic and Neuropsychiatric Disorders: the Iceberg Still Lies Beneath the Surface
1521-0081/71/3/383–412$35.00 https://doi.org/10.1124/pr.118.015487 PHARMACOLOGICAL REVIEWS Pharmacol Rev 71:383–412, July 2019 Copyright © 2019 by The Author(s) This is an open access article distributed under the CC BY-NC Attribution 4.0 International license. ASSOCIATE EDITOR: JEFFREY M. WITKIN 5-HT3 Receptor Antagonists in Neurologic and Neuropsychiatric Disorders: The Iceberg Still Lies beneath the Surface Gohar Fakhfouri,1 Reza Rahimian,1 Jonas Dyhrfjeld-Johnsen, Mohammad Reza Zirak, and Jean-Martin Beaulieu Department of Psychiatry and Neuroscience, Faculty of Medicine, CERVO Brain Research Centre, Laval University, Quebec, Quebec, Canada (G.F., R.R.); Sensorion SA, Montpellier, France (J.D.-J.); Department of Pharmacodynamics and Toxicology, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran (M.R.Z.); and Department of Pharmacology and Toxicology, University of Toronto, Toronto, Ontario, Canada (J.-M.B.) Abstract. ....................................................................................384 I. Introduction. ..............................................................................384 II. 5-HT3 Receptor Structure, Distribution, and Ligands.........................................384 A. 5-HT3 Receptor Agonists .................................................................385 B. 5-HT3 Receptor Antagonists. ............................................................385 Downloaded from 1. 5-HT3 Receptor Competitive Antagonists..............................................385 2. 5-HT3 Receptor -

Irritable Bowel Syndrome-Diarrhea
Clinical Pharmacy Program Guidelines for Irritable Bowel Syndrome-Diarrhea Program Prior Authorization Medication Lotronex (alosetron), Viberzi (eluxadoline) Markets in Scope Arizona, California, Colorado, Hawaii, Maryland, Nevada, New Jersey, New York, New York EPP, Pennsylvania- CHIP, Rhode Island, South Carolina Issue Date 3/2013 Pharmacy and 3/2021 Therapeutics Approval Date Effective Date 6/2021 1. Background: Lotronex (alosetron) is indicated only for use in women with severe diarrhea- predominant irritable bowel syndrome (IBS) who have: chronic IBS, had anatomical or biochemical abnormalities of the gastrointestinal tract excluded, and not responded adequately to conventional therapy. Viberzi (eluxadoline) is a mu-opioid receptor agonist, indicated for the treatment of irritable bowel syndrome with diarrhea (IBS-D) in adults. 2. Coverage Criteria: A. Lotronex 1. Initial Authorization a. Lotronex will be approved based on all of the following criteria: (1) Diagnosis of severe diarrhea-predominant irritable bowel syndrome (IBS) -AND- (2) Symptoms for at least 6 months -AND- (3) Patient was female at birth -AND- Confidential and Proprietary, © 2021 UnitedHealthcare Services Inc. 1 (4) Age greater than or equal to 18 years -AND- (5) History of failure, contraindication, or intolerance to a tricyclic antidepressant (e.g. amitriptyline) Authorization will be issued for 12 months. 2. Reauthorization a. Lotronex will be approved based on the following criterion: (1) Documentation of positive clinical response to Lotronex therapy Authorization will be issued for 12 months. B. Viberzi 1. Initial Authorization a. Viberzi will be approved based on all of the following criteria: (1) Diagnosis of irritable bowel syndrome with diarrhea (IBS-D) -AND- (2) History of failure, contraindication, or intolerance to a tricyclic antidepressant (e.g. -

IBS Treatment
TREATMENTS OF IBS Douglas A. Drossman, MD Co-Director UNC Center for Functional GI & Motility Disorders INTRODUCTION In recent years, there has been increased interest by physicians and the pharmaceutical industry regarding newer treatments for IBS. Before discussing these new treatments, it is important to consider the overall management strategy in IBS. This is necessary because patients with IBS exhibit a wide spectrum of symptoms of varying frequencies and degrees of severity. There is no one ideal treatment for IBS, and the newer medications may work best for only a subset of patients having this disorder. Therefore, the clinician must first apply certain general management approaches and, following this, treatment choices will depend on the nature (i.e., predominant diarrhea, constipation, or bloating, etc.) and severity (mild, moderate, severe) of the symptoms. The symptoms of IBS may have any of several underlying causes. These can include: (a) abnormal motility (uncoordinated or excessive contractions that can lead to diarrhea, constipation, bloating) (b) visceral hypersensitivity (lower pain threshold of the nerves that can produce abdominal discomfort or pain) resulting from the abnormal motility, stress or infection (c) dysfunction of the brain's ability to regulate these visceral (intestinal) activities. Treatments will vary depending on which of these possibilities are occurring. In general, milder symptoms relate primarily to abnormal motility, often in response to food, activity or stress, and/or visceral hypersensitivity. They are commonly treated symptomatically with pharmacological agents directed at the gut. However, more severe symptoms often relate to dysfunction of the brain-gut regulatory system with associated psychosocial effects, and psychological or behavioral treatments and antidepressants are frequently helpful. -

Tegaserod in the Treatment of Constipation-Predominant Functional Gastrointestinal Disorders
DRUG PROFILE Tegaserod in the treatment of constipation-predominant functional gastrointestinal disorders Anurag Agrawal & Irritable bowel syndrome is a common condition for which, until recently, treatment Peter Whorwell† options have been limited. Tegaserod has selective serotonin subtype 4 receptor agonist †Author for correspondence activity and acts by increasing gastrointestinal motility, secretion and possibly reducing ERC Building, First Floor, Wythenshawe Hospital, visceral sensitivity. It has been developed to treat patients with irritable bowel syndrome Southmoor Road, who suffer from abdominal pain, constipation and bloating. Studies so far suggest that it is Manchester, M23 9LT, an effective treatment for these symptoms with an excellent safety profile. Its role in other UK Tel.: +44 161 291 5813 functional gastrointestinal disorders, such as functional dyspepsia, is still being assessed. Fax: +44 161 291 4184 This review describes the structure, pharmacokinetic and pharmacodynamic properties of tegaserod and its effect on gastrointestinal physiology, as well as its clinical utility. Irritable bowel syndrome (IBS) is the most com- drugs such as antidiarrheals, laxatives, antispas- mon condition dealt with by gastroenterolo- modics and antidepressants. Behavioral therapy gists, accounting for up to 30% of their practice is sometimes tried in patients who do not and 10% of primary care case loads [1]. It is respond to conventional treatment. characterized by abdominal pain or discomfort In addition to its costs to the patient, IBS also often related to a change in bowel habit and fre- has a significant direct and indirect economic quently exacerbated by eating. Investigation burden. The direct cost in terms of healthcare reveals no structural abnormality, although a utilization has been estimated to be between variety of gastrointestinal (GI) physiological US$1.7–10 billion/year in the USA alone. -
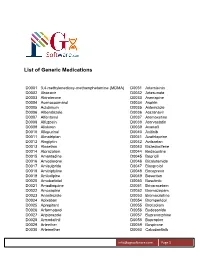
List of Generic Medications
List of Generic Medications D0001 3,4-methylenedioxy-methamphetamine (MDMA) D0031 Artemisinin D0002 Abacavir D0032 Artesunate D0003 Abiraterone D0033 Asenapine D0004 Acenocoumarol D0034 Aspirin D0005 Aclidinium D0035 Astemizole D0006 Albendazole D0036 Atazanavir D0007 Alfentanyl D0037 Atomoxetine D0008 Alfuzosin D0038 Atorvastatin D0009 Aliskiren D0039 Avanafil D0010 Allopurinol D0040 Axitinib D0011 Almotriptan D0041 Azathioprine D0012 Alogliptin D0042 Azilsartan D0013 Alosetron D0043 Bazedoxifene D0014 Alprazolam D0044 Bedaquiline D0015 Amantadine D0045 Bepridil D0016 Amiodarone D0046 Bicalutamide D0017 Amisulpride D0047 Bisoprolol D0018 Amitriptyline D0048 Boceprevir D0019 Amlodipine D0049 Bosentan D0020 Amobarbital D0050 Bosutinib D0021 Amodiaquine D0051 Brivaracetam D0022 Amoxapine D0052 Bromazepam D0023 Anastrozole D0053 Bromocriptine D0024 Apixaban D0054 Bromperidol D0025 Aprepitant D0055 Brotizolam D0026 Arformoterol D0056 Budesonide D0027 Aripiprazole D0057 Buprenorphine D0028 Armodafinil D0058 Bupropion D0029 Arteether D0059 Buspirone D0030 Artemether D0060 Cabozantinib [email protected] Page 1 List of Generic Medications D0061 Canagliflozin D0091 Clofarabine D0062 Cannabidiol (CBD) D0092 Clofibrate D0063 Cannabinol (CBN) D0093 Clomipramine D0064 Captopril D0094 Clonazepam D0065 Carbamazepine D0095 Clonidine D0066 Carisoprodol D0096 Clopidogrel D0067 Carmustine D0097 Clorazepate D0068 Carvedilol D0098 Clozapine D0069 Celecoxib D0099 Cocaine D0070 Ceritinib D0100 Codeine D0071 Cerivastatin D0101 Colchicine D0072 Cetirizine