IBS Treatment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Therapeutic Class Overview Irritable Bowel Syndrome Agents
Therapeutic Class Overview Irritable Bowel Syndrome Agents Therapeutic Class Overview/Summary: This review will focus on agents used for the treatment of Irritable Bowel Syndrome (IBS).1-5 IBS is a gastrointestinal syndrome characterized primarily by non-specific chronic abdominal pain, usually described as a cramp-like sensation, and abnormal bowel habits, either constipation or diarrhea, in which there is no organic cause. Other common gastrointestinal symptoms may include gastroesophageal reflux, dysphagia, early satiety, intermittent dyspepsia and nausea. Patients may also experience a wide range of non-gastrointestinal symptoms. Some notable examples include sexual dysfunction, dysmenorrhea, dyspareunia, increased urinary frequency/urgency and fibromyalgia-like symptoms.6 IBS is defined by one of four subtypes. IBS with constipation (IBS-C) is the presence of hard or lumpy stools with ≥25% of bowel movements and loose or watery stools with <25% of bowel movements. When IBS is associated with diarrhea (IBS-D) loose or watery stools are present with ≥25% of bowel movements and hard or lumpy stools are present with <25% of bowel movements. Mixed IBS (IBS-M) is defined as the presence of hard or lumpy stools with ≥25% and loose or water stools with ≥25% of bowel movements. Final subtype, or unsubtyped, is all other cases of IBS that do not fall into the other classes. Pharmacological therapy for IBS depends on subtype.7 While several over-the-counter or off-label prescription agents are used for the treatment of IBS, there are currently only two agents approved by the Food and Drug Administration (FDA) for the treatment of IBS-C and three agents approved by the FDA for IBS-D. -

Prophylactic Antiemetic Therapy with Ondansetron, Granisetron and Metoclopramide in Patients Undergoing Laparoscopic Cholecystectomy Under GA
JK SCIENCE ORIGINAL ARTICLE Prophylactic Antiemetic Therapy with Ondansetron, Granisetron and Metoclopramide in Patients Undergoing Laparoscopic Cholecystectomy Under GA Vishal Gupta, Renu Wakhloo, Anjali Mehta, Satya Dev Gupta Abstract The aim of the present study was to compare the antiemetic effect of intravenous Granisetron, Ondansetron & Metoclopramide in a randomized blinded study for prophylaxis of post operative nausea and vomiting (PONV) in patients undergoing laparoscopic cholecystectomy under general anaesthesia. 60 patients (ASA I & II) undergoing laparoscopic cholecystectomy under general anaesthesia were randomly allocated into three equal groups (n=20). Emetic episodes in first 24 hours were recorded and compared in different study groups. Results were analyzed. Minimal emetic episodes were observed in early post-operative period (1-12hrs) in patients who had received intravenous granisetron in comparison to ondansetron and metoclopramide. However, after 12 hours emesis free periods were statistically insignificant between group A and B while patients in group C had no antiemetic effect. Keywords Post Operative Nausea and Vomiting (PONV), Granisetron, Ondensetron, Metoclopramide Introduction The most common and distressing symptoms, which vascular anastomoses and increased intracranial follow anaesthesia and surgery, are pain and emesis. The pressure(4). The anaesthetic consequences are aspiration syndrome of nausea, retching and vomiting is known as pneumonitis and discomfort in recovery. For institutions 'sickness' and each part of it can be distinguished as a there is increased financial burden because of increased separate entity (1). PONV (post operative nausea and nursing care, delayed discharge from Phase I and II vomiting) has been characterized as big 'little problem(2) recovery units and unexpected admissions. Hence, and has been a common complication for both in patients prophylactic antiemetic therapy is needed for all these and out patients undergoing virtually all types of surgical patients. -

Comprehensive Pgx Report for 1 / 31 Examples of Different Levels of Evidence for Pgx Snps
Comprehensive PGx report for PERSONAL DETAILS Advanced Diagnostics Laboratory LLC CLIA:31D2149403 Phone: Fax: PATIENT DOB Address: 1030 North Kings Highway Suite 304 Cherry Hill, NJ 08034 GENDER FEMALE Website: http://advanceddiagnosticslaboratory.com/ SPECIMEN TYPE Oral Fluid LABORATORY INFORMATION ORDERING PHYSICIAN ACCESSION NUMBER 100344 FACILITY COLLECTION DATE 08/10/2020 RECEIVED DATE 08/14/2020 REPORT GENERATED 09/08/2020 LABORATORY DIRECTOR Dr. Jeanine Chiaffarano Current Patient Medication Clonidine (Catapres, Kapvay) The personalized pharmacogenomics profile of this patient reveals intermediate CYP2D6-mediated metabolism, extensive CYP1A2-mediated metabolism, and extensive CYP3A5-mediated metabolism. For further details, please find supporting evidence in this report or on websites such as www.pharmgkb.org or www.fda.gov. Losartan (Cozaar) The personalized pharmacogenomics profile of this patient reveals extensive CYP2C9-mediated metabolism, extensive CYP3A4-mediated metabolism, and extensive CYP3A5-mediated metabolism. For further details, please find supporting evidence in this report or on websites such as www.pharmgkb.org or www.fda.gov. Diltiazem (Cardizem, Tiazac) The personalized pharmacogenomics profile of this patient reveals extensive CYP3A4-mediated metabolism, intermediate CYP2C19-mediated metabolism, and extensive CYP3A5- mediated metabolism. For further details, please find supporting evidence in this report or on websites such as www.pharmgkb.org or www.fda.gov. Labetalol (Normodyne, Trandate) The personalized pharmacogenomics profile of this patient reveals intermediate CYP2D6-mediated metabolism, and intermediate CYP2C19-mediated metabolism. For further details, please find supporting evidence in this report or on websites such as www.pharmgkb.org or www.fda.gov. Mycophenolate mofetil (Myfortic, CellCept) The personalized pharmacogenomics profile of this patient reveals extensive CYP3A4-mediated metabolism, extensive CYP3A5-mediated metabolism, and extensive CYP2C8-mediated metabolism. -

Muscarinic Acetylcholine Receptor
mAChR Muscarinic acetylcholine receptor mAChRs (muscarinic acetylcholine receptors) are acetylcholine receptors that form G protein-receptor complexes in the cell membranes of certainneurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibersin the parasympathetic nervous system. mAChRs are named as such because they are more sensitive to muscarine than to nicotine. Their counterparts are nicotinic acetylcholine receptors (nAChRs), receptor ion channels that are also important in the autonomic nervous system. Many drugs and other substances (for example pilocarpineand scopolamine) manipulate these two distinct receptors by acting as selective agonists or antagonists. Acetylcholine (ACh) is a neurotransmitter found extensively in the brain and the autonomic ganglia. www.MedChemExpress.com 1 mAChR Inhibitors & Modulators (+)-Cevimeline hydrochloride hemihydrate (-)-Cevimeline hydrochloride hemihydrate Cat. No.: HY-76772A Cat. No.: HY-76772B Bioactivity: Cevimeline hydrochloride hemihydrate, a novel muscarinic Bioactivity: Cevimeline hydrochloride hemihydrate, a novel muscarinic receptor agonist, is a candidate therapeutic drug for receptor agonist, is a candidate therapeutic drug for xerostomia in Sjogren's syndrome. IC50 value: Target: mAChR xerostomia in Sjogren's syndrome. IC50 value: Target: mAChR The general pharmacol. properties of this drug on the The general pharmacol. properties of this drug on the gastrointestinal, urinary, and reproductive systems and other… gastrointestinal, urinary, and reproductive systems and other… Purity: >98% Purity: >98% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 10mM x 1mL in DMSO, Size: 10mM x 1mL in DMSO, 1 mg, 5 mg 1 mg, 5 mg AC260584 Aclidinium Bromide Cat. No.: HY-100336 (LAS 34273; LAS-W 330) Cat. -

(Orion) 5 Mg Tablets Buspirone (Orion) 10 Mg Tablets
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Buspirone (Orion) 5 mg tablets Buspirone (Orion) 10 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 5 mg buspirone hydrochloride. Each tablet contains 10 mg buspirone hydrochloride. Excipient with known effect: 5 mg tablet: Each tablet contains 59.5 mg lactose (as monohydrate) 10 mg tablet: Each tablet contains 118.9 mg lactose (as monohydrate). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet. 5 mg tablet: White or almost white, oval tablets debossed with ‘ORN 30’ on one side and a score on the other side. The tablet can be divided into equal doses. 10 mg tablet: White or almost white, oval tablets debossed with ‘ORN 31’ on one side and a score on the other side. The tablet can be divided into equal doses. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Buspirone hydrochloride is indicated for the management of anxiety with or without accompanying depression in adults. Buspirone hydrochloride is indicated for the management of anxiety disorders or the short- term relief of symptoms of anxiety with or without accompanying depression. 4.2 Posology and method of administration The usual starting dose is 5 mg given three times daily. This may be titrated according to the needs of the patient and the daily dose increased by 5 mg increments every two or three days depending upon the therapeutic response to a maximum daily dose of 60 mg. After dosage titration the usual daily dose will be 20 to 30 mg per day in divided doses. -

MODERN INDICATIONS for the USE of OPIPRAMOL Krzysztof Krysta1, Sławomir Murawiec2, Anna Warchala1, Karolina Zawada3, Wiesław J
Psychiatria Danubina, 2015; Vol. 27, Suppl. 1, pp 435–437 Conference paper © Medicinska naklada - Zagreb, Croatia MODERN INDICATIONS FOR THE USE OF OPIPRAMOL Krzysztof Krysta1, Sławomir Murawiec2, Anna Warchala1, Karolina Zawada3, Wiesław J. Cubała4, Mariusz S. Wiglusz4, Katarzyna Jakuszkowiak-Wojten4, Marek Krzystanek5 & Irena Krupka-Matuszczyk1 1Department of Psychiatry and Psychotherapy, Medical University of Silesia, Katowice, Poland 2“Dialogue” Therapy Centre, Warsaw, Poland 3Department of Pneumonology, Medical University of Silesia, Katowice, Poland 4Department of Psychiatry, Medical University of Gdańsk, Gdańsk, Poland 5Department of Rehabilitation Psychiatry, Medical University of Silesia, Katowice, Poland SUMMARY Opipramol is considered as a pharmacological agent that does not fit the classification taking into account the division of antidepressants, antipsychotics and anxiolytics. It has a structure related to tricyclic antidepressants but it has a different mechanism of action, i.e. binding to sigma1 and to sigma2 sites. It has been regarded as an effective drug in general anxiety disorders together with other agents like SSRI`s, SNRI`s, buspirone and pregabalin for many years. It can however also be indicated in other conditions, e.g. it may be used as a premedication in the evening prior to surgery, positive results are also observed in psychopharmacological treatment with opipramol in somatoform disorders, symptoms of depression can be significantly reduced in the climacteric syndrome. The latest data from literature present also certain dangers and side effects, which may result due to opipramol administration. Mania may be induced not only in bipolar patients treated with opipramol, but it can be an adverse drug reaction in generalized anxiety disorder. This analysis shows however that opipramol is an important drug still very useful in different clinical conditions. -

The Role of Antispasmodics in Managing Irritable Bowel Syndrome
DOI: https://doi.org/10.22516/25007440.309 Review articles The role of antispasmodics in managing irritable bowel syndrome Valeria Atenea Costa Barney,1* Alan Felipe Ovalle Hernández.1 1 Internal Medicine and Gastroenterology specialist Abstract in San Ignacio University Hospital, Pontificia Universidad Javeriana, Bogotá, Colombia. Although antispasmodics are the cornerstone of treating irritable bowel syndrome, there are a number of an- tispasmodic medications currently available in Colombia. Since they are frequently used to treat this disease, *Correspondence: [email protected] we consider an evaluation of them to be important. ......................................... Received: 26/10/18 Keywords Accepted: 11/02/19 Antispasmodic, irritable bowel syndrome, pinaverium bromide, otilonium bromide, Mebeverin, trimebutine. INTRODUCTION consistency. The criteria must be met for three consecutive months prior to diagnosis and symptoms must have started Irritable bowel syndrome (IBS) is one of the most fre- a minimum of six months before diagnosis. (3, 4) quent chronic gastrointestinal functional disorders. It is There are no known structural or anatomical explanations characterized by recurrent abdominal pain associated with of the pathophysiology of IBS and its exact cause remains changes in the rhythm of bowel movements with either or unknown. Nevertheless, several mechanisms have been both constipation and diarrhea. Swelling and bloating are proposed. Altered gastrointestinal motility may contribute frequent occurrences. (1) to changes in bowel habits reported by some patients, and a IBS is divided into two subtypes: predominance of cons- combination of smooth muscle spasms, visceral hypersen- tipation (20-30% of patients) and predominance of dia- sitivity and abnormalities of central pain processing may rrhea (20-30% of patients). -

Inappropriate Elimination Disorders
INAPPROPRIATE ELIMINATION DISORDERS What is “inappropriate elimination”? This is a term that means that a cat is urinating and/or defecating in the house but not in the litter box. What causes it? After medical causes of these problems have been ruled out, the source of the problem is considered a behavioral disorder. Behavioral causes of inappropriate elimination fall into two general categories: 1) a dislike of the litter box, and 2) stress-related misbehavior. Why would a cat not like its litter box? One of the main reasons for this is because the litter box has become objectionable to the cat. This usually occurs because it is not cleaned frequently enough or because the cat does not like the litter in it. The latter is called substrate aversion; it can occur because the litter was changed to a new, objectionable type or because the cat just got tired of the old litter. What stresses can cause inappropriate elimination? There are probably hundreds of these, but the more common ones are as follows: a) A new person (especially a baby) in the house b) A person that has recently left the house (permanently or temporarily) c) New furniture d) New drapes e) New carpet f) Rearrangement of the furniture g) Moving to a new house h) A new pet in the house i) A pet that has recently left the house j) A new cat in the neighborhood that can be seen by the indoor cat k) A cat in “heat” in the neighborhood l) A new dog in the neighborhood that can be heard by the indoor cat I feel that this is a problem that cannot be tolerated, even if the cat has to leave my house. -

Clinical Practice Guidelines for Irritable Bowel Syndrome in Korea, 2017 Revised Edition
J Neurogastroenterol Motil, Vol. 24 No. 2 April, 2018 pISSN: 2093-0879 eISSN: 2093-0887 https://doi.org/10.5056/jnm17145 JNM Journal of Neurogastroenterology and Motility Review Clinical Practice Guidelines for Irritable Bowel Syndrome in Korea, 2017 Revised Edition Kyung Ho Song,1,2 Hye-Kyung Jung,3* Hyun Jin Kim,4 Hoon Sup Koo,1 Yong Hwan Kwon,5 Hyun Duk Shin,6 Hyun Chul Lim,7 Jeong Eun Shin,6 Sung Eun Kim,8 Dae Hyeon Cho,9 Jeong Hwan Kim,10 Hyun Jung Kim11; and The Clinical Practice Guidelines Group Under the Korean Society of Neurogastroenterology and Motility 1Department of Internal Medicine, Konyang University College of Medicine, Daejeon, Korea; 2Konyang University Myunggok Medical Research Institute Daejeon, Korea; 3Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea; 4Department of Internal Medicine, Gyeongsang National University, College of Medicine, Jinju, Korea; 5Department of Internal Medicine, Kyungpook National University, School of Medicine, Daegu, Korea; 6Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea; 7Department of Internal Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea; 8Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea; 9Department of Internal Medicine, Sungkyunkwan University School of Medicine, Changwon, Korea; 10Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Korea; and 11Department of Preventive Medicine, Korea University College of Medicine, Seoul, Korea In 2011, the Korean Society of Neurogastroenterology and Motility (KSNM) published clinical practice guidelines on the management of irritable bowel syndrome (IBS) based on a systematic review of the literature. The KSNM planned to update the clinical practice guidelines to support primary physicians, reduce the socioeconomic burden of IBS, and reflect advances in the pathophysiology and management of IBS. -

5-HT3 Receptor Antagonists in Neurologic and Neuropsychiatric Disorders: the Iceberg Still Lies Beneath the Surface
1521-0081/71/3/383–412$35.00 https://doi.org/10.1124/pr.118.015487 PHARMACOLOGICAL REVIEWS Pharmacol Rev 71:383–412, July 2019 Copyright © 2019 by The Author(s) This is an open access article distributed under the CC BY-NC Attribution 4.0 International license. ASSOCIATE EDITOR: JEFFREY M. WITKIN 5-HT3 Receptor Antagonists in Neurologic and Neuropsychiatric Disorders: The Iceberg Still Lies beneath the Surface Gohar Fakhfouri,1 Reza Rahimian,1 Jonas Dyhrfjeld-Johnsen, Mohammad Reza Zirak, and Jean-Martin Beaulieu Department of Psychiatry and Neuroscience, Faculty of Medicine, CERVO Brain Research Centre, Laval University, Quebec, Quebec, Canada (G.F., R.R.); Sensorion SA, Montpellier, France (J.D.-J.); Department of Pharmacodynamics and Toxicology, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran (M.R.Z.); and Department of Pharmacology and Toxicology, University of Toronto, Toronto, Ontario, Canada (J.-M.B.) Abstract. ....................................................................................384 I. Introduction. ..............................................................................384 II. 5-HT3 Receptor Structure, Distribution, and Ligands.........................................384 A. 5-HT3 Receptor Agonists .................................................................385 B. 5-HT3 Receptor Antagonists. ............................................................385 Downloaded from 1. 5-HT3 Receptor Competitive Antagonists..............................................385 2. 5-HT3 Receptor -

Pharmaceutical Appendix to the Harmonized Tariff Schedule
Harmonized Tariff Schedule of the United States (2019) Revision 13 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2019) Revision 13 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -
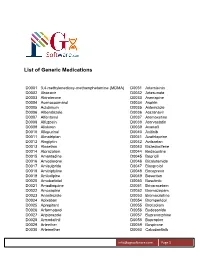
List of Generic Medications
List of Generic Medications D0001 3,4-methylenedioxy-methamphetamine (MDMA) D0031 Artemisinin D0002 Abacavir D0032 Artesunate D0003 Abiraterone D0033 Asenapine D0004 Acenocoumarol D0034 Aspirin D0005 Aclidinium D0035 Astemizole D0006 Albendazole D0036 Atazanavir D0007 Alfentanyl D0037 Atomoxetine D0008 Alfuzosin D0038 Atorvastatin D0009 Aliskiren D0039 Avanafil D0010 Allopurinol D0040 Axitinib D0011 Almotriptan D0041 Azathioprine D0012 Alogliptin D0042 Azilsartan D0013 Alosetron D0043 Bazedoxifene D0014 Alprazolam D0044 Bedaquiline D0015 Amantadine D0045 Bepridil D0016 Amiodarone D0046 Bicalutamide D0017 Amisulpride D0047 Bisoprolol D0018 Amitriptyline D0048 Boceprevir D0019 Amlodipine D0049 Bosentan D0020 Amobarbital D0050 Bosutinib D0021 Amodiaquine D0051 Brivaracetam D0022 Amoxapine D0052 Bromazepam D0023 Anastrozole D0053 Bromocriptine D0024 Apixaban D0054 Bromperidol D0025 Aprepitant D0055 Brotizolam D0026 Arformoterol D0056 Budesonide D0027 Aripiprazole D0057 Buprenorphine D0028 Armodafinil D0058 Bupropion D0029 Arteether D0059 Buspirone D0030 Artemether D0060 Cabozantinib [email protected] Page 1 List of Generic Medications D0061 Canagliflozin D0091 Clofarabine D0062 Cannabidiol (CBD) D0092 Clofibrate D0063 Cannabinol (CBN) D0093 Clomipramine D0064 Captopril D0094 Clonazepam D0065 Carbamazepine D0095 Clonidine D0066 Carisoprodol D0096 Clopidogrel D0067 Carmustine D0097 Clorazepate D0068 Carvedilol D0098 Clozapine D0069 Celecoxib D0099 Cocaine D0070 Ceritinib D0100 Codeine D0071 Cerivastatin D0101 Colchicine D0072 Cetirizine