Medicago Spp. As Potential Sources of Bioactive Isoflavones
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Relation Structure/Activité De Tanins Bioactifs Contre Les Nématodes
En vue de l'obtention du DOCTORAT DE L'UNIVERSITÉ DE TOULOUSE Délivré par : Institut National Polytechnique de Toulouse (INP Toulouse) Discipline ou spécialité : Pathologie, Toxicologie, Génétique et Nutrition Présentée et soutenue par : Mme JESSICA QUIJADA PINANGO le jeudi 17 décembre 2015 Titre : RELATION STRUCTURE/ACTIVITE DE TANINS BIOACTIFS CONTRE LES NEMATODES GASTROINTESTINAUX (HAEMONCHUS CONTORTUS) PARASITES DES PETITS RUMINANTS Ecole doctorale : Sciences Ecologiques, Vétérinaires, Agronomiques et Bioingénieries (SEVAB) Unité de recherche : Interactions Hôtes - Agents Pathogènes (IHAP) Directeur(s) de Thèse : M. HERVÉ HOSTE Rapporteurs : M. ADIBE LUIZ ABDALLA, UNIVERSIDAD DE SAO PAULO Mme HEIDI ENEMARK, NORWEGIAN VETERINARY INSTITUTE Membre(s) du jury : 1 M. FRANÇOIS SCHELCHER, ECOLE NATIONALE VETERINAIRE DE TOULOUSE, Président 2 M. HERVÉ HOSTE, INRA TOULOUSE, Membre 2 Mme CARINE MARIE-MAGDELAINE, INRA PETIT BOURG, Membre 2 M. SMARO SOTIRAKI, HAO-DEMETER, Membre 2 M. VINCENT NIDERKORN, INRA CLERMONT FERRAND, Membre QUIJADA J. 2015 Cette thèse est dédiée à mes parents, Teresa et Héctor, À mon mari, Rafäel, pour son soutien inconditionnel, son amour illimité, sa patience, sa loyauté, son amitié et surtout sa confidence, À ma grand-mère, Marcolina, car m'ait donné le plus grand et précieux cadeau en ma vie : ma foi en Dieu ma forteresse et mon espoir (Isaïas 41:13). À mes adorés sœurs, belle- sœurs et frère : Yurlin, Indira, Iskay, Olga, Zoraida et Jesus. Merci pour l’amour infini que m’ont toujours été donné, celui qu’a été prolongé par l'amour de mes merveilleux neveux. 1 QUIJADA J. 2015 REMERCIEMENTS Je remercie tout d’abord mon Dieu pour me donner le cadeau de la vie, et la forteresse pour vivre chaque jour. -

IN SILICO ANALYSIS of FUNCTIONAL Snps of ALOX12 GENE and IDENTIFICATION of PHARMACOLOGICALLY SIGNIFICANT FLAVONOIDS AS
Tulasidharan Suja Saranya et al. Int. Res. J. Pharm. 2014, 5 (6) INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 – 8407 Research Article IN SILICO ANALYSIS OF FUNCTIONAL SNPs OF ALOX12 GENE AND IDENTIFICATION OF PHARMACOLOGICALLY SIGNIFICANT FLAVONOIDS AS LIPOXYGENASE INHIBITORS Tulasidharan Suja Saranya, K.S. Silvipriya, Manakadan Asha Asokan* Department of Pharmaceutical Chemistry, Amrita School of Pharmacy, Amrita Viswa Vidyapeetham University, AIMS Health Sciences Campus, Kochi, Kerala, India *Corresponding Author Email: [email protected] Article Received on: 20/04/14 Revised on: 08/05/14 Approved for publication: 22/06/14 DOI: 10.7897/2230-8407.0506103 ABSTRACT Cancer is a disease affecting any part of the body and in comparison with normal cells there is an elevated level of lipoxygenase enzyme in different cancer cells. Thus generation of lipoxygenase enzyme inhibitors have suggested being valuable. Individual variation was identified by the functional effects of Single Nucleotide Polymorphisms (SNPs). 696 SNPs were identified from the ALOX12 gene, out of which 73 were in the coding non-synonymous region, from which 8 were found to be damaging. In silico analysis was performed to determine naturally occurring flavonoids such as isoflavones having the basic 3- phenylchromen-4-one skeleton for the pharmacological activity, like Genistein, Diadzein, Irilone, Orobol and Pseudobaptigenin. O-methylated isoflavones such as Biochanin, Calycosin, Formononetin, Glycitein, Irigenin, 5-O-Methylgenistein, Pratensein, Prunetin, ψ-Tectorigenin, Retusin and Tectorigenine were also used for the study. Other natural products like Aesculetin, a coumarin derivative; flavones such as ajoene and baicalein were also used for the comparative study of these natural compounds along with acteoside and nordihydroguaiaretic acid (antioxidants) and active inhibitors like Diethylcarbamazine, Zileuton and Azelastine as standard for the computational analysis. -

Phylogenetic Insights on the Isoflavone Profile Variations In
Food Research International 76 (2015) 51–57 Contents lists available at ScienceDirect Food Research International journal homepage: www.elsevier.com/locate/foodres Phylogenetic insights on the isoflavone profile variations in Fabaceae spp.: Assessment through PCA and LDA Tatiana Visnevschi-Necrasov a,b, João C.M. Barreira b,c,⁎,SaraC.Cunhab, Graça Pereira d, Eugénia Nunes a, M. Beatriz P.P. Oliveira b a CIBIO-ICETA, Faculdade de Ciências, Universidade do Porto, R. Padre Armando Quintas 4485-661 Vairão, Portugal b REQUIMTE, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto, Rua Jorge Viterbo Ferreira, No. 228, 4050-313, Porto,Portugal c CIMO-ESA, Instituto Politécnico de Bragança, Campus de Santa Apolónia, Apartado 1172, 5301-855 Bragança, Portugal d INRB/IP — INIA — Instituto Nacional de Recursos Biológicos, Caia E São Pedro Estrada Gil Vaz, 7350-228 Elvas, Portugal article info abstract Article history: Legumes (Fabaceae) are important crops, known as sources of food, feed for livestock and raw materials for in- Received 30 September 2014 dustry. Their ability to capture atmospheric nitrogen during symbiotic processes with soil bacteria reduces the Received in revised form 15 November 2014 need for expensive chemical fertilizers, improving soil and water quality. Several Fabaceae species are acknowl- Accepted 20 November 2014 edged for the high levels of secondary metabolites. Isoflavones are among the most well-known examples of Available online 28 November 2014 these compounds, being recognized for their several types of biological activity. Herein, isoflavone profiles were characterized in nine species of four Fabaceae genera (Biserrula, Lotus, Ornithopus and Scorpiurus). Plants Chemical compounds studied in this article: fl Daidzin (PubChem CID: 107971) were harvested in the late ower physiological stage to prevent biased results due to naturally occurring varia- Genistin (PubChem CID: 5281377) tions along the vegetative cycle. -

Ep 3138585 A1
(19) TZZ¥_¥_T (11) EP 3 138 585 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.03.2017 Bulletin 2017/10 A61L 27/20 (2006.01) A61L 27/54 (2006.01) A61L 27/52 (2006.01) (21) Application number: 16191450.2 (22) Date of filing: 13.01.2011 (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • Gousse, Cecile GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO 74230 Dingy Saint Clair (FR) PL PT RO RS SE SI SK SM TR • Lebreton, Pierre Designated Extension States: 74000 Annecy (FR) BA ME •Prost,Nicloas 69440 Mornant (FR) (30) Priority: 13.01.2010 US 687048 26.02.2010 US 714377 (74) Representative: Hoffmann Eitle 30.11.2010 US 956542 Patent- und Rechtsanwälte PartmbB Arabellastraße 30 (62) Document number(s) of the earlier application(s) in 81925 München (DE) accordance with Art. 76 EPC: 15178823.9 / 2 959 923 Remarks: 11709184.3 / 2 523 701 This application was filed on 29-09-2016 as a divisional application to the application mentioned (71) Applicant: Allergan Industrie, SAS under INID code 62. 74370 Pringy (FR) (54) STABLE HYDROGEL COMPOSITIONS INCLUDING ADDITIVES (57) The present specification generally relates to hydrogel compositions and methods of treating a soft tissue condition using such hydrogel compositions. EP 3 138 585 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 138 585 A1 Description CROSS REFERENCE 5 [0001] This patent application is a continuation-in-part of U.S. -

Systems Approaches and Polypharmacology for Drug Discovery from Herbal Medicines: an Example Using Licorice
Journal of Ethnopharmacology 146 (2013) 773–793 Contents lists available at SciVerse ScienceDirect Journal of Ethnopharmacology journal homepage: www.elsevier.com/locate/jep Systems approaches and polypharmacology for drug discovery from herbal medicines: An example using licorice Hui Liu a, Jinan Wang a, Wei Zhou a, Yonghua Wang a,n, Ling Yang b a Bioinformatics Center, College of Life Sciences, Northwest A&F University, Yangling, Shaanxi 712100, China b Lab of Pharmaceutical Resource Discovery, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning 116023, China article info abstract Article history: Ethnopharmacological relevance: Licorice, one of the oldest and most popular herbal medicines in the Received 5 September 2012 world, has been widely used in traditional Chinese medicine as a cough reliever, anti-inflammatory, Received in revised form anti-anabrosis, immunomodulatory, anti-platelet, antiviral (hepatitis) and detoxifying agent. Licorice 3 February 2013 was used as an example to show drug discovery from herbal drugs using systems approaches and Accepted 4 February 2013 polypharmacology. Available online 14 February 2013 Aim of the study: Herbal medicines are becoming more mainstream in clinical practice and show value Keywords: in treating and preventing diseases. However, due to its extreme complexity both in chemical Licorice components and mechanisms of action, deep understanding of botanical drugs is still difficult. Thus, Chinese herbal medicine a comprehensive systems approach which could identify active ingredients and their targets in the Systems pharmacology crude drugs and more importantly, understand the biological basis for the pharmacological properties Network analysis Drug discovery of herbal medicines is necessary. Materials and methods: In this study, a novel systems pharmacology model that integrates oral bioavailability screening, drug-likeness evaluation, blood–brain barrier permeation, target identifica- tion and network analysis has been established to investigate the herbal medicines. -

Induction of Prenylated Isoflavonoids and Stilbenoids in Legumes
Induction of prenylated isoflavonoids and stilbenoids in legumes Siti Aisyah Thesis committee Promotor Prof. Dr H. Gruppen Professor of Food Chemistry Wageningen University Co-promotor Dr J.-P. Vincken Associate professor, Laboratory of Food Chemistry Wageningen University Other members Prof. Dr P.J.G.M. de Wit, Wageningen University Prof. Dr E.J. Smid, Wageningen University Prof. Dr S. de Saeger, Ghent University, Belgium Dr H.W.M. Hilhorst, Wageningen University This research was conducted under the auspices of the Graduate School VLAG (Advanced studies in Food Technology, Agrobiotechnology, Nutrition and Health Sciences). Induction of prenylated isoflavonoids and stilbenoids in legumes Siti Aisyah Thesis submitted in fulfilment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. Dr A. P. J. Mol, in the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Tuesday 13 October 2015 at 11 a.m. in the Aula. Siti Aisyah Induction of prenylated isoflavonoids and stilbenoids in legumes 156 pages. PhD thesis, Wageningen University, Wageningen, NL (2015) With references, with summary in English ISBN: 978-94-6257-481-6 Abstract The germination of legume seeds in the presence or absence of stress factors was studied with respect to compositional changes in prenylated isoflavonoids and stilbenoids. Different strategies were applied using (i) different types of legume seed, (ii) different stress factors i.e. biotic, abiotic and their combination, and (iii) different time point of application of the fungus. Mass spectrometric tools to better characterize the position of prenyl groups in the molecules were optimized. -

PDF-Document
Supplementary Materials 1 Metabolomics profiling analysis of red clover Table S1. Compound identification of red clover in positive and negative ion modes. MS1 No RT Ion Ion Measur Predicte Ste Le Flow Err MS2 MS3 Identification . min Form Formula ed d m af er ppm m/z m/z [M − 195.050 1 1.43 C6H11O7 195.0499 2.82 Gluconic acid ++ ++ +++ H]- 5 179.03506(C9H7O4); Benzoylcitronensa [M − 295.045 295.0448 ++ 2 4.46 C13H11O8 0.67 133.01451(C4H5O5); [295-179]135.04524(C8H7O2); ure ++ +++ H]- 0 4 + 115.00400(C4H3O4); [M + 355.102 ++ 3 5.42 C16H19O9 355.1024 1.21 chlorogenic acid + +++ H]+ 8 + [433-271]253.04956(C15H9O4); 243.06529(C14H11O4); [M + 433.111 Genistein- ++ 4 6.60 C21H21O10 433.1129 −3.54 271.06067(C15H11O5); 215.07042(C13H11O3); +++ +++ H]+ 4 glucoside + 153.01839(C7H5O4); 149.02335(C8H5O3); [M + 417.117 5 6.71 C21H21O9 417.1180 −2.34 239.07021(C15H11O3) daidzin + ++ +++ H]+ 0 [M + 447.127 calycosin-7-O-β-D- ++ 6 7.20 C22H23O10 447.1286 −1.91 269.08072(C16H13O4) +++ +++ H]+ 7 glucoside + 1 [533-285]270.05258(C15H10O5); Calycosin-7-O-β- [M + 533.127 253.04982(C15H9O4); ++ 7 8.03 C25H25O13 533.1290 −3.54 285.07642(C16H13O5); D-glucoside 4′′-O- +++ +++ H]+ 1 225.05478(C14H9O3); + malonate 137.02333(C7H5O3); [M + 433.112 ++ 8 8.95 C21H21O10 433.1129 −2.11 255.06516(C15H11O4) genistin ++ +++ H]+ 0 + [519-271]253.04958(C15H9O4,); Genistein- 243.06526(C14H11O4); [M + 519.111 433.11377(C21H21O10); glucoside ++ 9 9.31 C24H23O13 519.1133 −3.25 215.07045(C13H11O3); - +++ H]+ 6 271.06073(C15H11O5); malonate + 153.01833(C7H5O4); 149.02333(C8H5O3); -

A Journey of Twenty-Five Years Through the Ecological Biochemistry of Flavonoidsthis Review Was Written in Response to the Auth
70028 (RV-8) Biosci. Biotechnol. Biochem., 71, 70028-1–18, 2007 Award Review A Journey of Twenty-Five Years through the Ecological Biochemistry of Flavonoids Satoshi TAHARA Laboratory of Ecological Chemistry, Graduate School of Agriculture, Hokkaido University, Kita-ku, Sapporo 060-8589, Japan Online Publication, June 7, 2007 [doi:10.1271/bbb.70028] The ecological biochemistry of flavonoids, in which I 3 have been engaged for 25 years, is summarized in this 2 3 1 review article. The review covers (1) a survey of rare 1 2 bio-active flavonoids in higher plants; (2) the fungal metabolism of prenylated flavonoids; (3) flavonoids anti- Flavonoid skeleton Isoflavonoid skeleton in the narrow sense (1,2-diphenylpropane) doting against benzimidazole fungicides; (4) dihydro- (1,3-diphenylpropane) flavonolAdvance ampelopsin in Salix sacharinensis as a View feeding stimulant towards willow beetles; and (5) flavones as Flavonoid skeletons in the broad sense signaling substances in the life-cycle development of the 3' 8 1 2' 9 O phytopathogenic Peronosporomycete Aphanomyces co- 4' 7 2 chlioides, a cause of spinach root rot and sugar beet 8 1 1' B A C 2' 9 O 5' 6 1' 7 10 3 3' damping-off diseases. Finally recent trends in the eco- A C 2 6' 5 4 B 6 3 4' logical biochemistry of flavonoids are briefly described. 10 6' 5 4 5' 2-phenylchroman 3-phenylchroman Key words: flavonoids; ecological biochemistry; secon- dary metabolites; signal substance; allelo- Fig. 1. ProofsCarbon Skeletons of Flavonoids. chemicals skeletons that can be subdivided into some 10 classes I. General Introduction according to their oxidation levels and variations in the complexity of carbon skeletons caused by alk(en)yl- One of the representative classes of plant secondary ation, glycosylation, oligomerization, and so on. -
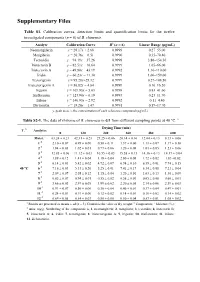
Supplementary Files
Supplementary Files Table S1. Calibration curves, detection limits and quantification limits for the twelve investigated components (n = 6) of B. chinensis. Analyte Calibration Curve R2 (n = 6) Linear Range (μg/mL) Neomangiferin y = 28.17x + 2.68 0.9999 0.27–55.00 Mangiferin y = 20.76x − 6.51 0.9990 0.35–70.40 Tectoridin y = 94.19x − 37.26 0.9998 3.86–154.50 Iristectorin B y = 82.51x − 10.64 0.9999 1.65–66.00 Iristectorin A y = 49.80x − 43.19 0.9992 1.10–110.00 Iridin y = 60.23x − 11.30 0.9999 1.06–159.00 Tectorigenin y = 95.28x+25.32 0.9999 0.27–108.50 Iristectorigenin A y = 56.82x − 4.84 0.9999 0.16–16.20 Irigenin y = 109.93x + 5.03 0.9998 0.83–41.60 Irisflorentin y = 123.90x − 0.19 0.9993 0.23–11.70 Irilone y = 146.93x − 2.92 0.9992 0.11–4.40 Dictomitin y = 18.20x − 1.47 0.9991 0.59–17.70 y, peak area; x, the concentration of each reference compound (μg/mL). Table S2-1. The data of rhizome of B. chinensis in G1 from different sampling points at 40 °C. a Drying Time (min) T. b Analytes 0 120 240 360 480 600 Moist. c 63.24 ± 0.21 42.31 ± 0.23 27.25 ± 0.06 20.14 ± 0.10 12.04 ± 0.13 8.13 ± 0.06 1 d 2.10 ± 0.07 0.95 ± 0.00 0.50 ± 0.11 1.37 ± 0.00 1.11 ± 0.07 1.17 ± 0.10 2 d 1.84 ± 0.03 1.02 ± 0.01 0.77 ± 0.06 1.20 ± 0.08 1.03 ± 0.05 1.23 ± 0.06 3 d 12.85 ± 0.06 11.12 ± 0.03 10.55 ± 0.03 15.58 ± 0.13 14.36 ± 0.13 14.17 ± 0.04 4 d 1.89 ± 0.12 1.41 ± 0.04 1.19 ± 0.04 2.00 ± 0.08 1.72 ± 0.02 1.83 ±0.02 5 d 6.14 ± 0.03 5.42 ± 0.02 4.72 ± 0.07 6.94 ± 0.10 6.59 ± 0.01 7.74 ± 0.15 40 °C 6 d 7.16 ± 0.03 5.33 ± 0.26 5.25 ± 0.01 7.41 ± 0.17 6.34 ± -

The Α-Glucosidase Inhibiting Isoflavones Isolated from Belamcanda Chinensis Leaf Extract
ORIGINAL ARTICLE Rec. Nat. Prod . 6:2 (2012) 110-120 The α-Glucosidase Inhibiting Isoflavones Isolated from Belamcanda chinensis Leaf Extract Chongming Wu 1,3†, Jingyao Shen 1† , Pingping He 1† , Yan Chen 1,2 , Liang Li 1, 1 1 1 1 1,2* 1,2* Liang Zhang ,Ye Li , Yujuan Fu , Rongji Dai , Weiwei Meng and Yulin Deng 1School of Life Science, Beijing Institute of Technology, Beijing 100081, PR China 2Beijing BIT&GY Pharmaceutical R&D, Beijing 100081, PR China 3Protein Science Laboratory of Ministry of Education, School of Life Sciences, Tsinghua University, Beijing 100084, PR China (Received March 29, 2011; Revised October 5, 2011; Accepted October 14, 2011) Abstract: The dried rhizome of Belamcanda chinensis is an important Chinese traditional medicine used for the treatment of inflammation and many other disorders. Previously, we reported the hypo- and antihyper-glycemic effects of the aqueous leaf extract of B. chinensis (BCL) and identified the isoflavones as its principal active fraction. In the present study, the α-glucosidase inhibitory effect of BCL and its rough isoflavone preparation (BIF) was tested in vitro and in vivo . Thirteen isoflavones were isolated from BCL and their α-glucosidase inhibitory activity was screened in vitro . The results showed that BCL (500 and 1000 mg/kg) and BIF (250 and 500 mg/kg) greatly inhibited the increase in blood glucose level after 5 g/kg starch loading in normal mice. Six out of the thirteen isoflavones (swertisin, 2”-O-rhamnosylswertisin, genistein, genistin, mangiferin and daidzin) exhibited strong α-glucosidase inhibitory activity in vitro . HPLC analysis showed that swertisin was the most abundant isoflavone in BCL accounting for 1.24% of BCL, 7.44% of BIF, and 11.24% of the total isoflavone fraction of BCL, respectively. -

Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Tuberculosis
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Tuberculosis Chemical Activity Count (+)-3-HYDROXY-9-METHOXYPTEROCARPAN 1 (+)-8HYDROXYCALAMENENE 1 (+)-ALLOMATRINE 1 (+)-ALPHA-VINIFERIN 3 (+)-AROMOLINE 1 (+)-CASSYTHICINE 1 (+)-CATECHIN 10 (+)-CATECHIN-7-O-GALLATE 1 (+)-CATECHOL 1 (+)-CEPHARANTHINE 1 (+)-CYANIDANOL-3 1 (+)-EPIPINORESINOL 1 (+)-EUDESMA-4(14),7(11)-DIENE-3-ONE 1 (+)-GALBACIN 2 (+)-GALLOCATECHIN 3 (+)-HERNANDEZINE 1 (+)-ISOCORYDINE 2 (+)-PSEUDOEPHEDRINE 1 (+)-SYRINGARESINOL 1 (+)-SYRINGARESINOL-DI-O-BETA-D-GLUCOSIDE 2 (+)-T-CADINOL 1 (+)-VESTITONE 1 (-)-16,17-DIHYDROXY-16BETA-KAURAN-19-OIC 1 (-)-3-HYDROXY-9-METHOXYPTEROCARPAN 1 (-)-ACANTHOCARPAN 1 (-)-ALPHA-BISABOLOL 2 (-)-ALPHA-HYDRASTINE 1 Chemical Activity Count (-)-APIOCARPIN 1 (-)-ARGEMONINE 1 (-)-BETONICINE 1 (-)-BISPARTHENOLIDINE 1 (-)-BORNYL-CAFFEATE 2 (-)-BORNYL-FERULATE 2 (-)-BORNYL-P-COUMARATE 2 (-)-CANESCACARPIN 1 (-)-CENTROLOBINE 1 (-)-CLANDESTACARPIN 1 (-)-CRISTACARPIN 1 (-)-DEMETHYLMEDICARPIN 1 (-)-DICENTRINE 1 (-)-DOLICHIN-A 1 (-)-DOLICHIN-B 1 (-)-EPIAFZELECHIN 2 (-)-EPICATECHIN 6 (-)-EPICATECHIN-3-O-GALLATE 2 (-)-EPICATECHIN-GALLATE 1 (-)-EPIGALLOCATECHIN 4 (-)-EPIGALLOCATECHIN-3-O-GALLATE 1 (-)-EPIGALLOCATECHIN-GALLATE 9 (-)-EUDESMIN 1 (-)-GLYCEOCARPIN 1 (-)-GLYCEOFURAN 1 (-)-GLYCEOLLIN-I 1 (-)-GLYCEOLLIN-II 1 2 Chemical Activity Count (-)-GLYCEOLLIN-III 1 (-)-GLYCEOLLIN-IV 1 (-)-GLYCINOL 1 (-)-HYDROXYJASMONIC-ACID 1 (-)-ISOSATIVAN 1 (-)-JASMONIC-ACID 1 (-)-KAUR-16-EN-19-OIC-ACID 1 (-)-MEDICARPIN 1 (-)-VESTITOL 1 (-)-VESTITONE 1 -

Evaluation of Firefly and Renilla Luciferase Inhibition In
International Journal of Molecular Sciences Article Evaluation of Firefly and Renilla Luciferase Inhibition in Reporter-Gene Assays: A Case of Isoflavonoids Maša Kenda 1,†, Jan Vegelj 1,†, Barbara Herlah 1,2, Andrej Perdih 1,2 , PˇremyslMladˇenka 3 and Marija Sollner Dolenc 1,* 1 Faculty of Pharmacy, University of Ljubljana, AškerˇcevaCesta 7, 1000 Ljubljana, Slovenia; [email protected] (M.K.); [email protected] (J.V.); [email protected] (B.H.); [email protected] (A.P.) 2 National Institute of Chemistry, Hajdrihova 19, 1000 Ljubljana, Slovenia 3 Faculty of Pharmacy in Hradec Králové, Charles University, Akademika Heyrovského 1203, 500 05 Hradec Králové, Czech Republic; [email protected] * Correspondence: [email protected]; Tel.: +386-1-476-9572 † Co-first author, these authors contributed equally to this work. Abstract: Firefly luciferase is susceptible to inhibition and stabilization by compounds under investi- gation for biological activity and toxicity. This can lead to false-positive results in in vitro cell-based assays. However, firefly luciferase remains one of the most commonly used reporter genes. Here, we evaluated isoflavonoids for inhibition of firefly luciferase. These natural compounds are often studied using luciferase reporter-gene assays. We used a quantitative structure–activity relationship (QSAR) model to compare the results of in silico predictions with a newly developed in vitro assay that enables concomitant detection of inhibition of firefly and Renilla luciferases. The QSAR model Citation: Kenda, M.; Vegelj, J.; predicted a moderate to high likelihood of firefly luciferase inhibition for all of the 11 isoflavonoids Herlah, B.; Perdih, A.; Mladˇenka,P.; investigated, and the in vitro assays confirmed this for seven of them: daidzein, genistein, glycitein, Sollner Dolenc, M.