Relation Structure/Activité De Tanins Bioactifs Contre Les Nématodes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flavonoid Glucodiversification with Engineered Sucrose-Active Enzymes Yannick Malbert
Flavonoid glucodiversification with engineered sucrose-active enzymes Yannick Malbert To cite this version: Yannick Malbert. Flavonoid glucodiversification with engineered sucrose-active enzymes. Biotechnol- ogy. INSA de Toulouse, 2014. English. NNT : 2014ISAT0038. tel-01219406 HAL Id: tel-01219406 https://tel.archives-ouvertes.fr/tel-01219406 Submitted on 22 Oct 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Last name: MALBERT First name: Yannick Title: Flavonoid glucodiversification with engineered sucrose-active enzymes Speciality: Ecological, Veterinary, Agronomic Sciences and Bioengineering, Field: Enzymatic and microbial engineering. Year: 2014 Number of pages: 257 Flavonoid glycosides are natural plant secondary metabolites exhibiting many physicochemical and biological properties. Glycosylation usually improves flavonoid solubility but access to flavonoid glycosides is limited by their low production levels in plants. In this thesis work, the focus was placed on the development of new glucodiversification routes of natural flavonoids by taking advantage of protein engineering. Two biochemically and structurally characterized recombinant transglucosylases, the amylosucrase from Neisseria polysaccharea and the α-(1→2) branching sucrase, a truncated form of the dextransucrase from L. Mesenteroides NRRL B-1299, were selected to attempt glucosylation of different flavonoids, synthesize new α-glucoside derivatives with original patterns of glucosylation and hopefully improved their water-solubility. -

IN SILICO ANALYSIS of FUNCTIONAL Snps of ALOX12 GENE and IDENTIFICATION of PHARMACOLOGICALLY SIGNIFICANT FLAVONOIDS AS
Tulasidharan Suja Saranya et al. Int. Res. J. Pharm. 2014, 5 (6) INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 – 8407 Research Article IN SILICO ANALYSIS OF FUNCTIONAL SNPs OF ALOX12 GENE AND IDENTIFICATION OF PHARMACOLOGICALLY SIGNIFICANT FLAVONOIDS AS LIPOXYGENASE INHIBITORS Tulasidharan Suja Saranya, K.S. Silvipriya, Manakadan Asha Asokan* Department of Pharmaceutical Chemistry, Amrita School of Pharmacy, Amrita Viswa Vidyapeetham University, AIMS Health Sciences Campus, Kochi, Kerala, India *Corresponding Author Email: [email protected] Article Received on: 20/04/14 Revised on: 08/05/14 Approved for publication: 22/06/14 DOI: 10.7897/2230-8407.0506103 ABSTRACT Cancer is a disease affecting any part of the body and in comparison with normal cells there is an elevated level of lipoxygenase enzyme in different cancer cells. Thus generation of lipoxygenase enzyme inhibitors have suggested being valuable. Individual variation was identified by the functional effects of Single Nucleotide Polymorphisms (SNPs). 696 SNPs were identified from the ALOX12 gene, out of which 73 were in the coding non-synonymous region, from which 8 were found to be damaging. In silico analysis was performed to determine naturally occurring flavonoids such as isoflavones having the basic 3- phenylchromen-4-one skeleton for the pharmacological activity, like Genistein, Diadzein, Irilone, Orobol and Pseudobaptigenin. O-methylated isoflavones such as Biochanin, Calycosin, Formononetin, Glycitein, Irigenin, 5-O-Methylgenistein, Pratensein, Prunetin, ψ-Tectorigenin, Retusin and Tectorigenine were also used for the study. Other natural products like Aesculetin, a coumarin derivative; flavones such as ajoene and baicalein were also used for the comparative study of these natural compounds along with acteoside and nordihydroguaiaretic acid (antioxidants) and active inhibitors like Diethylcarbamazine, Zileuton and Azelastine as standard for the computational analysis. -

Revisiting Greek Propolis: Chromatographic Analysis and Antioxidant Activity Study
RESEARCH ARTICLE Revisiting Greek Propolis: Chromatographic Analysis and Antioxidant Activity Study Konstantinos M. Kasiotis1*, Pelagia Anastasiadou1, Antonis Papadopoulos2, Kyriaki Machera1* 1 Benaki Phytopathological Institute, Department of Pesticides Control and Phytopharmacy, Laboratory of Pesticides' Toxicology, Kifissia, Athens, Greece, 2 Benaki Phytopathological Institute, Department of Phytopathology, Laboratory of Non-Parasitic Diseases, Kifissia, Athens, Greece * [email protected] (KMK); [email protected] (KM) Abstract a1111111111 Propolis is a bee product that has been extensively used in alternative medicine and recently a1111111111 a1111111111 has gained interest on a global scale as an essential ingredient of healthy foods and cosmet- a1111111111 ics. Propolis is also considered to improve human health and to prevent diseases such as a1111111111 inflammation, heart disease, diabetes and even cancer. However, the claimed effects are anticipated to be correlated to its chemical composition. Since propolis is a natural product, its composition is consequently expected to be variable depending on the local flora align- ment. In this work, we present the development of a novel HPLC-PDA-ESI/MS targeted OPEN ACCESS method, used to identify and quantify 59 phenolic compounds in Greek propolis hydroalco- holic extracts. Amongst them, nine phenolic compounds are herein reported for the first time Citation: Kasiotis KM, Anastasiadou P, Papadopoulos A, Machera K (2017) Revisiting in Greek propolis. Alongside GC-MS complementary analysis was employed, unveiling Greek Propolis: Chromatographic Analysis and eight additional newly reported compounds. The antioxidant activity study of the propolis Antioxidant Activity Study. PLoS ONE 12(1): samples verified the potential of these extracts to effectively scavenge radicals, with the e0170077. doi:10.1371/journal.pone.0170077 extract of Imathia region exhibiting comparable antioxidant activity to that of quercetin. -

2.1 Structure Elucidation 14 2.2 Synthesis 15
, HIERDIE EKSEMPlAAR MAG ONDER University Free State GEEN OMSTANDIGHEDE UIT DIE mml~lmmMMllllllln34300000347405 Universiteit Vrystaat BIBLIOTEEK VER\VYDER WORD NIE THE STRUCTURE AND SYNTHESIS OF OLIGOFLA VANOIDS AND OLIGOSTILBENES FROM CASSIA ABBREVIATA. Dissertation submitted infulfilment of the requirements for the degree Master of Science in the Department of Chemisrty Faculty of Natural Sciences at University of Orange Free State Bloemfontein by Makhosazana Claribel Mthembu Supervisor: Prof. E. Malan Co-supervisor Dr J. C. Coetzee JANUARY 2000 ACKNOWLEDGEMENTS I wish to express my gratitude to the following people: • God the Almighty for the love and support he has shown me throughout my studies. • My supervisors Professor f. Malan and Dr j. C. Coetzee, their guidance and helpful recommendations throughout this study will always be appreciated: • • Proff. Malan for his constructive guidance, critism and invaluable support in putting this work in paper. • Dr j. C. Coetzeefor recording my NMR spectra, his encourangement and unselfish assistance. • Proff. V. Brandt ,D. Ferrdra and NRF sponsor for granting me this opportumity. • Finally the members of the fomily and [riends, Irene,jaqui, Zanele, Vusl and Busi for their love, tolerance and constant encouragement, I owe the greatest debt to them. M. C. Mthembu Table of Content Abstract LITERATURE SURVERY CHAPTER 1: MONOMERIC STILBENES 1 1.1 Nomenclature 1 1.2 Structure and Natural Occurrence 3 1.3 Structure Elucidation 5 1.4 Synthesis 5 CHAPTER 2: OLIGOMERIC STILBENES 11 2.1 Structure Elucidation 14 2.2 Synthesis 15 CHAPTER 3.: FLA VAN-3-0LS 16 3.1 Nomenclature 16 3.2 Structure and Natural Occurrence 17 3.3 Structure Elucidation 19 CHAPTER 4: LEUCOANTHOCY ANIDINS. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0268722 A1 Siegelin Et Al
US 20110268722A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0268722 A1 Siegelin et al. (43) Pub. Date: Nov. 3, 2011 (54) COMBINATION THERAPIES WITH Publication Classification MTOCHONORAL-TARGETED (51) Int. Cl ANT-TUMIORAGENTS A638/17 (2006.01) CI2N 5/071 (2010.01) (76) Inventors: Markus D. Siegelin, Weehawken, A6IP35/00 (2006.01) NJ (US); Dario C. Altieri, A 6LX 39/395 (2006.01) Worcester, MA (US) C07D 225/06 (2006.01) (52) U.S. Cl. ..................... 424/130.1; 514/18.9; 540/461; (21) Appl. No.: 13/092,475 435/375 (57) ABSTRACT (22) Filed: Apr. 22, 2011 Described are mitochondria-targeted anti-tumor agents, death receptor agonists, autophagy inhibitors, and NF-KB Related U.S. Application Data signaling pathway inhibitors, and methods of making and (60) Provisional application No. 61/326,872, filed on Apr. using the same for the treatment of disorders associated with 22, 2010. unwanted cell proliferation. Patent Application Publication Nov. 3, 2011 Sheet 1 of 13 US 2011/0268722 A1 120% 8: 8: 8 CE 60% : 5 P_GA s : RA 2:: : P-GA-RA : : 8 N3 FAS Figure 1A Patent Application Publication Nov. 3, 2011 Sheet 2 of 13 US 2011/0268722 A1 igure 1B ---, igure 2A Figure 2B Patent Application Publication Nov. 3, 2011 Sheet 3 of 13 US 2011/0268722 A1 8. s::::::: se: -83.383.3 ---sixxxxx:----- --~~~x-x-x-xxii. : s: 8 i88 s: sig ::::::: ...i Figure 2C Figure 2D Figure 3A Figure 3B Patent Application Publication Nov. 3, 2011 Sheet 4 of 13 US 2011/0268722 A1 ::: :*:: & : : isix; {S3.g3-8 : Exx8:8-3 : {kari 333333. -

Phylogenetic Insights on the Isoflavone Profile Variations In
Food Research International 76 (2015) 51–57 Contents lists available at ScienceDirect Food Research International journal homepage: www.elsevier.com/locate/foodres Phylogenetic insights on the isoflavone profile variations in Fabaceae spp.: Assessment through PCA and LDA Tatiana Visnevschi-Necrasov a,b, João C.M. Barreira b,c,⁎,SaraC.Cunhab, Graça Pereira d, Eugénia Nunes a, M. Beatriz P.P. Oliveira b a CIBIO-ICETA, Faculdade de Ciências, Universidade do Porto, R. Padre Armando Quintas 4485-661 Vairão, Portugal b REQUIMTE, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto, Rua Jorge Viterbo Ferreira, No. 228, 4050-313, Porto,Portugal c CIMO-ESA, Instituto Politécnico de Bragança, Campus de Santa Apolónia, Apartado 1172, 5301-855 Bragança, Portugal d INRB/IP — INIA — Instituto Nacional de Recursos Biológicos, Caia E São Pedro Estrada Gil Vaz, 7350-228 Elvas, Portugal article info abstract Article history: Legumes (Fabaceae) are important crops, known as sources of food, feed for livestock and raw materials for in- Received 30 September 2014 dustry. Their ability to capture atmospheric nitrogen during symbiotic processes with soil bacteria reduces the Received in revised form 15 November 2014 need for expensive chemical fertilizers, improving soil and water quality. Several Fabaceae species are acknowl- Accepted 20 November 2014 edged for the high levels of secondary metabolites. Isoflavones are among the most well-known examples of Available online 28 November 2014 these compounds, being recognized for their several types of biological activity. Herein, isoflavone profiles were characterized in nine species of four Fabaceae genera (Biserrula, Lotus, Ornithopus and Scorpiurus). Plants Chemical compounds studied in this article: fl Daidzin (PubChem CID: 107971) were harvested in the late ower physiological stage to prevent biased results due to naturally occurring varia- Genistin (PubChem CID: 5281377) tions along the vegetative cycle. -

Polymerization Degrees, Molecular Weights and Protein-Binding Affinities of Condensed Tannin Fractions from a Leucaena Leucocephala Hybrid
Molecules 2014, 19, 7990-8010; doi:10.3390/molecules19067990 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Polymerization Degrees, Molecular Weights and Protein-Binding Affinities of Condensed Tannin Fractions from a Leucaena leucocephala Hybrid Mookiah Saminathan 1, Hui Yin Tan 2, Chin Chin Sieo 1,3, Norhani Abdullah 3,4, Clemente Michael Vui Ling Wong 5, Emilia Abdulmalek 6 and Yin Wan Ho 1,* 1 Institute of Bioscience, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia; E-Mails: [email protected] (M.S.); [email protected] (C.C.S.) 2 Faculty of Applied Sciences and Computing, Tunku Abdul Rahman University College, 53300 Kuala Lumpur, Malaysia; E-Mail: [email protected] 3 Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia; E-Mail: [email protected] 4 Institute of Tropical Agriculture, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia 5 Biotechnology Research Institute, Universiti Malaysia Sabah, Jalan UMS, 88400 Kota Kinabalu, Sabah, Malaysia; E-Mail: [email protected] 6 Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +603-8947-2161; Fax: +603-8947-2101. Received: 18 March 2014; in revised form: 11 May 2014 / Accepted: 21 May 2014 / Published: 12 June 2014 Abstract: Condensed tannins (CTs) form insoluble complexes with proteins and are able to protect them from degradation, which could lead to rumen bypass proteins. Depending on their degrees of polymerization (DP) and molecular weights, CT fractions vary in their capability to bind proteins. -

WO 2011/086458 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date _ . ... _ 21 July 2011 (21.07.2011) WO 2011/086458 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61L 27/20 (2006.01) A61L 27/54 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (21) International Application Number: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, PCT/IB20 11/000052 DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 13 January 201 1 (13.01 .201 1) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (26) Publication Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 12/687,048 13 January 2010 (13.01 .2010) US (84) Designated States (unless otherwise indicated, for every 12/714,377 26 February 2010 (26.02.2010) US kind of regional protection available): ARIPO (BW, GH, 12/956,542 30 November 2010 (30.1 1.2010) us GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, (71) Applicant (for all designated States except US): AL- TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, LERGAN INDUSTRIE, SAS [FR/FR]; Route de EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, Promery, Zone Artisanale de Pre-Mairy, F-74370 Pringy LV, MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, (FR). -

Ep 3138585 A1
(19) TZZ¥_¥_T (11) EP 3 138 585 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.03.2017 Bulletin 2017/10 A61L 27/20 (2006.01) A61L 27/54 (2006.01) A61L 27/52 (2006.01) (21) Application number: 16191450.2 (22) Date of filing: 13.01.2011 (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • Gousse, Cecile GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO 74230 Dingy Saint Clair (FR) PL PT RO RS SE SI SK SM TR • Lebreton, Pierre Designated Extension States: 74000 Annecy (FR) BA ME •Prost,Nicloas 69440 Mornant (FR) (30) Priority: 13.01.2010 US 687048 26.02.2010 US 714377 (74) Representative: Hoffmann Eitle 30.11.2010 US 956542 Patent- und Rechtsanwälte PartmbB Arabellastraße 30 (62) Document number(s) of the earlier application(s) in 81925 München (DE) accordance with Art. 76 EPC: 15178823.9 / 2 959 923 Remarks: 11709184.3 / 2 523 701 This application was filed on 29-09-2016 as a divisional application to the application mentioned (71) Applicant: Allergan Industrie, SAS under INID code 62. 74370 Pringy (FR) (54) STABLE HYDROGEL COMPOSITIONS INCLUDING ADDITIVES (57) The present specification generally relates to hydrogel compositions and methods of treating a soft tissue condition using such hydrogel compositions. EP 3 138 585 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 138 585 A1 Description CROSS REFERENCE 5 [0001] This patent application is a continuation-in-part of U.S. -

PDF-Document
Supplementary Materials 1 Metabolomics profiling analysis of red clover Table S1. Compound identification of red clover in positive and negative ion modes. MS1 No RT Ion Ion Measur Predicte Ste Le Flow Err MS2 MS3 Identification . min Form Formula ed d m af er ppm m/z m/z [M − 195.050 1 1.43 C6H11O7 195.0499 2.82 Gluconic acid ++ ++ +++ H]- 5 179.03506(C9H7O4); Benzoylcitronensa [M − 295.045 295.0448 ++ 2 4.46 C13H11O8 0.67 133.01451(C4H5O5); [295-179]135.04524(C8H7O2); ure ++ +++ H]- 0 4 + 115.00400(C4H3O4); [M + 355.102 ++ 3 5.42 C16H19O9 355.1024 1.21 chlorogenic acid + +++ H]+ 8 + [433-271]253.04956(C15H9O4); 243.06529(C14H11O4); [M + 433.111 Genistein- ++ 4 6.60 C21H21O10 433.1129 −3.54 271.06067(C15H11O5); 215.07042(C13H11O3); +++ +++ H]+ 4 glucoside + 153.01839(C7H5O4); 149.02335(C8H5O3); [M + 417.117 5 6.71 C21H21O9 417.1180 −2.34 239.07021(C15H11O3) daidzin + ++ +++ H]+ 0 [M + 447.127 calycosin-7-O-β-D- ++ 6 7.20 C22H23O10 447.1286 −1.91 269.08072(C16H13O4) +++ +++ H]+ 7 glucoside + 1 [533-285]270.05258(C15H10O5); Calycosin-7-O-β- [M + 533.127 253.04982(C15H9O4); ++ 7 8.03 C25H25O13 533.1290 −3.54 285.07642(C16H13O5); D-glucoside 4′′-O- +++ +++ H]+ 1 225.05478(C14H9O3); + malonate 137.02333(C7H5O3); [M + 433.112 ++ 8 8.95 C21H21O10 433.1129 −2.11 255.06516(C15H11O4) genistin ++ +++ H]+ 0 + [519-271]253.04958(C15H9O4,); Genistein- 243.06526(C14H11O4); [M + 519.111 433.11377(C21H21O10); glucoside ++ 9 9.31 C24H23O13 519.1133 −3.25 215.07045(C13H11O3); - +++ H]+ 6 271.06073(C15H11O5); malonate + 153.01833(C7H5O4); 149.02333(C8H5O3); -

48003 Hughes Et Al 2017 Accepted Version.Pdf
ResearchOnline@JCU This is the Accepted Version of a paper published in the Journal: Critical Reviews in Food Science and Nutrition Hughes, Samuel D., Ketheesan, Natkunam, and Haleagrahara, Nagaraja (2017) The therapeutic potential of plant flavonoids on rheumatoid arthritis. Critical Reviews in Food Science and Nutrition, 57 (17). pp. 3601-3613. http://dx.doi.org/10.1080/10408398.2016.1246413 © 2015. This manuscript version is made available under the CC-BY-NC-ND 4.0 license http://creativecommons.org/licenses/by-nc-nd/4.0/ Critical Reviews in Food Science and Nutrition ISSN: 1040-8398 (Print) 1549-7852 (Online) Journal homepage: http://www.tandfonline.com/loi/bfsn20 The Therapeutic Potential of Plant Flavonoids on Rheumatoid Arthritis Samuel D. Hughes, Natkunam Ketheesan & Nagaraja Haleagrahara To cite this article: Samuel D. Hughes, Natkunam Ketheesan & Nagaraja Haleagrahara (2016): The Therapeutic Potential of Plant Flavonoids on Rheumatoid Arthritis, Critical Reviews in Food Science and Nutrition, DOI: 10.1080/10408398.2016.1246413 To link to this article: http://dx.doi.org/10.1080/10408398.2016.1246413 Accepted author version posted online: 22 Nov 2016. Submit your article to this journal Article views: 122 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=bfsn20 Download by: [JAMES COOK UNIVERSITY] Date: 23 March 2017, At: 16:37 ACCEPTED MANUSCRIPT The Therapeutic Potential of Plant Flavonoids on Rheumatoid Arthritis Samuel D. Hughes1, Natkunam Ketheesan2 & Nagaraja Haleagrahara3,* 1,2,3Biomedicine, College of Public Health, Medical & Veterinary Sciences, and 2, 3Australian Institute of Tropical Health and Medicine (AITHM), James Cook University, Townsville, Australia *Address correspondence to: Nagaraja Haleagrahara, Biomedicine, College of Public Health, Medical & Veterinary Sciences, James Cook University, Townsville, Australia. -
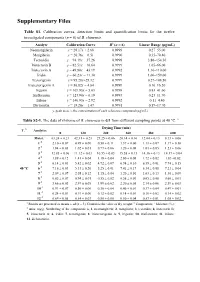
Supplementary Files
Supplementary Files Table S1. Calibration curves, detection limits and quantification limits for the twelve investigated components (n = 6) of B. chinensis. Analyte Calibration Curve R2 (n = 6) Linear Range (μg/mL) Neomangiferin y = 28.17x + 2.68 0.9999 0.27–55.00 Mangiferin y = 20.76x − 6.51 0.9990 0.35–70.40 Tectoridin y = 94.19x − 37.26 0.9998 3.86–154.50 Iristectorin B y = 82.51x − 10.64 0.9999 1.65–66.00 Iristectorin A y = 49.80x − 43.19 0.9992 1.10–110.00 Iridin y = 60.23x − 11.30 0.9999 1.06–159.00 Tectorigenin y = 95.28x+25.32 0.9999 0.27–108.50 Iristectorigenin A y = 56.82x − 4.84 0.9999 0.16–16.20 Irigenin y = 109.93x + 5.03 0.9998 0.83–41.60 Irisflorentin y = 123.90x − 0.19 0.9993 0.23–11.70 Irilone y = 146.93x − 2.92 0.9992 0.11–4.40 Dictomitin y = 18.20x − 1.47 0.9991 0.59–17.70 y, peak area; x, the concentration of each reference compound (μg/mL). Table S2-1. The data of rhizome of B. chinensis in G1 from different sampling points at 40 °C. a Drying Time (min) T. b Analytes 0 120 240 360 480 600 Moist. c 63.24 ± 0.21 42.31 ± 0.23 27.25 ± 0.06 20.14 ± 0.10 12.04 ± 0.13 8.13 ± 0.06 1 d 2.10 ± 0.07 0.95 ± 0.00 0.50 ± 0.11 1.37 ± 0.00 1.11 ± 0.07 1.17 ± 0.10 2 d 1.84 ± 0.03 1.02 ± 0.01 0.77 ± 0.06 1.20 ± 0.08 1.03 ± 0.05 1.23 ± 0.06 3 d 12.85 ± 0.06 11.12 ± 0.03 10.55 ± 0.03 15.58 ± 0.13 14.36 ± 0.13 14.17 ± 0.04 4 d 1.89 ± 0.12 1.41 ± 0.04 1.19 ± 0.04 2.00 ± 0.08 1.72 ± 0.02 1.83 ±0.02 5 d 6.14 ± 0.03 5.42 ± 0.02 4.72 ± 0.07 6.94 ± 0.10 6.59 ± 0.01 7.74 ± 0.15 40 °C 6 d 7.16 ± 0.03 5.33 ± 0.26 5.25 ± 0.01 7.41 ± 0.17 6.34 ±