B Lymphocyte Priming
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Priming of Virus-Immune Memory T Cells in Newborn Mice DAVID H
INFECTION AND IMMUNITY, Jan. 1984, p. 202-205 Vol. 43, No. 1 0019-9567/84/010202-04$02.00/0 Copyright (© 1984, American Society for Microbiology Priming of Virus-Immune Memory T Cells in Newborn Mice DAVID H. SCHWARTZ, JULIA L. HURWITZ,* NEIL S. GREENSPAN,t AND PETER C. DOHERTYt The Wistar Institute ofAnatomy and Biology, Philadelphia, Pennsylvania 19104 Received 6 May 1983/Accepted 27 September 1983 Neonatal BALB/c mice can be primed at birth by intravenous inoculation of a small dose of A/Puerto Rico/8/34 (H1N1) (PR8) influenza virus, UV-inactivated PR8 virus, or PR8 virus complexed with monoclonal antibody to give a secondary cytotoxic T lymphocyte response when restimulated in vitro as adults. The frequency of responding T cells after secondary stimulation in vitro is approximately 40% of that found for adult mice primed intraperitoneally with a large dose of PR8 virus. The majority of the T cells generated from mice primed at birth or as adults are cross-reactive for H-2-compatible targets infected with the PR8 (H1N1) or A/Hong Kong/X31 (H3N2) viruses. Splenocytes from neonates receiving UV-inactivated vaccinia virus at birth give an augmented secondary cytotoxic T lymphocyte response when restimulated 8 days later in adoptive irradiated adult hosts. We found no indications of specific immunological unrespon- siveness in mice exposed to either virus. We have analyzed previously the development of virus- cells per ml for 1 h at 37°C. Graded numbers of effector specific T cell competence in neonatal mice and have spleen cells and 2 x 105 syngeneic virus-infected, irradiated reported the presence of vaccinia virus-specific cytotoxic T stimulator cells were distributed in 100-.pl portions to the 60 lymphocyte (CTL) precursors in the thymus of 0- to 3-day- inside wells of 96-well, V-bottom plates. -

Priming with Inflammatory Cytokines Is Not a Prerequisite to Increase
Lange-Consiglio et al. Stem Cell Research & Therapy (2020) 11:99 https://doi.org/10.1186/s13287-020-01611-z RESEARCH Open Access Priming with inflammatory cytokines is not a prerequisite to increase immune- suppressive effects and responsiveness of equine amniotic mesenchymal stromal cells Anna Lange-Consiglio1* , Pietro Romele2, Marta Magatti2, Antonietta Silini2, Antonella Idda1, Nicola Antonio Martino3, Fausto Cremonesi1 and Ornella Parolini2,4 Abstract Background: Equine amniotic mesenchymal stromal cells (AMSCs) and their conditioned medium (CM) were evaluated for their ability to inhibit in vitro proliferation of peripheral blood mononuclear cells (PBMCs) with and without priming. Additionally, AMSC immunogenicity was assessed by expression of MHCI and MHCII and their ability to counteract the in vitro inflammatory process. Methods: Horse PBMC proliferation was induced with phytohemagglutinin. AMSC priming was performed with 10 ng/ml of TNF-α, 100 ng/ml of IFN-γ, and a combination of 5 ng/ml of TNF-α and 50 ng/ml of IFN-γ. The CM generated from naïve unprimed and primed AMSCs was also tested to evaluate its effects on equine endometrial cells in an in vitro inflammatory model induced by LPS. Immunogenicity marker expression (MHCI and II) was evaluated by qRT-PCR and by flow cytometry. Results: Priming does not increase MHCI and II expression. Furthermore, the inhibition of PBMC proliferation was comparable between naïve and conditioned cells, with the exception of AMSCs primed with both TNF-α and IFN-γ that had a reduced capacity to inhibit T cell proliferation. However, AMSC viability was lower after priming than under other experimental conditions. -
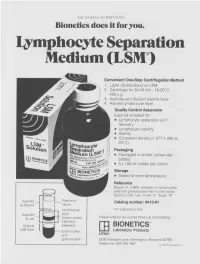
Lymphocyte Separation Medium (LSM
THE JOURNAL OF IMMUNOLOGY Bionetics does it for you. Lymphocyte Separation Medium (LSM wenient One-Step Centrifugation Method _ayer diluted blood on LSM. 2,entrifuge for 30-40 min., 18-20°C, ~.00 x g. ~,spirate and discard plasma layer -larvest lymphocyte layer. Quality Control Assurance Each lot is tested for: • Lymphocyte separation and recovery. • Lymphocyte viability. • Sterility. • Consistent density (1.077-1.080 at 20°C). Packaging • Packaged in amber, screw-cap bottles. • 5 x 100 ml bottles per carton. Storage. • Stored at room temperature. Reference Boyum, A. (1968): Isolation of mononuclear cells and granulocytes from human blood. Scand J. Clin. Lab. Invest. 21, Suppl. 97. Aspirate I IC[OI I IC~ LJI Catalog number: 8410-01 & discard serum Lymphocyte For Laboratory Use Aspirate layer & use (mononuclear Please write for our current Price List and Catalog. cells and Original platelets) ITi BIONETICS° LSM layer Erythrocytes Laboratory Products and Litton granulocytes 5516 Nicholson Lane, Kensington, Maryland 20795 Telephone: (301) 881-1557 1979 Litton Bionetics, tnc Get the most out of your high quality cytotoxic antibodies with LOW-TOX-M RABBIT COMPLEMENT LOW TOXICITY HIGH ACTIVITY Presentation: CL 3051 5 x 1 ml, lyophilized $30.00 When it comes to COMPLEMENT... come to CEDARLANE Direct orders or inquiries to: UNITED STATES: WORLDWIDE EXCEPT U.S. ,4 C~L CEDARLANE ACCURATE CHEMICAL & LABORATO RI ES SCIENTIFIC CORPORATION LIMITED 5516-8TH LINE, R.R. 2 28 TEC STREET, HICKSVtLLE, N.Y. 11801 HORNBY, ONTARIO, CANADA LOP 1E0 Telephone -

Stress and the Immune System Tracy B
4 World Health • 47th Yeor, No. 2, Morch-Aprill994 Stress and the immune system Tracy B. Herbert any people have the effects of factors as diverse as experienced the examinations, bereavement, divorce, Mconnection between stress unemployment, mental arithmetic, and getting sick. Colds, influenza, and looking after a relative with herpes and allergies seem worse Alzheimer's di sease. In general, when we are severely stressed at these studies find that stress is work or in the home. Others are related to changes in both the never sick until they go on vacation numbers of white blood cells in (that is, after the stress is over), and circulation and the quantity of then they spend the whole time antibody in the blood. Moreover, fighting the virus. Because of stress is associated with changes in intrinsic connections like these, the functioning of immune cells. many researchers are today That is, there is a relatively large exploring whether (and how) stress decrease in both lymphocyte and illness are actually linked. One proliferation and natural killer cell specific focus of this research is to activity in individuals who have study the effects of stress on the experienced stress. There seems to immune systems; after all, if stress A lymphocyte: stress may weaken the capacity be some connection between the affects immunity, that would be one of lymphocytes to combat infection. duration of the stress and the amount way in which stress could contribute of immune change. For example, the to illness. longer the stress, the greater the The function of the immune proliferation"- by incubating these decrease in the number of specific system is to protect us from cells for several days with types of white blood cells. -

Association Between Neutrophil-Lymphocyte Ratio and Herpes Zoster Infection in 1688 Living Donor Liver Transplantation Recipients at a Large Single Center
biomedicines Article Association between Neutrophil-Lymphocyte Ratio and Herpes Zoster Infection in 1688 Living Donor Liver Transplantation Recipients at a Large Single Center Ji-Hoon Sim, Young-Jin Moon, Sung-Hoon Kim, Kyoung-Sun Kim , Ju-Seung Lee, Jun-Gol Song * and Gyu-Sam Hwang Department of Anesthesiology and Pain Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea; [email protected] (J.-H.S.); [email protected] (Y.-J.M.); [email protected] (S.-H.K.); [email protected] (K.-S.K.); [email protected] (J.-S.L.); [email protected] (G.-S.H.) * Correspondence: [email protected]; Tel.: +82-2-3010-3869 Abstract: Liver transplantation (LT) is closely associated with decreased immune function, a contrib- utor to herpes zoster (HZ). However, risk factors for HZ in living donor LT (LDLT) remain unknown. Neutrophil-lymphocyte ratio (NLR) and immune system function are reportedly correlated. This study investigated the association between NLR and HZ in 1688 patients who underwent LDLT between January 2010 and July 2020 and evaluated risk factors for HZ and postherpetic neuralgia (PHN). The predictive power of NLR was assessed through the concordance index and an integrated discrimination improvement (IDI) analysis. Of the total cohort, 138 (8.2%) had HZ. The incidence of HZ after LT was 11.2 per 1000 person-years and 0.1%, 1.3%, 2.9%, and 13.5% at 1, 3, 5, and 10 years, Citation: Sim, J.-H.; Moon, Y.-J.; Kim, respectively. In the Cox regression analysis, preoperative NLR was significantly associated with HZ S.-H.; Kim, K.-S.; Lee, J.-S.; Song, J.-G.; (adjusted hazard ratio [HR], 1.05; 95% confidence interval [CI], 1.02–1.09; p = 0.005) and PHN (HR, Hwang, G.-S. -

Rapid Induction of Antigen-Specific CD4+ T Cells Guides Coordinated Humoral and Cellular Immune Responses to SARS-Cov-2 Mrna Vaccination
bioRxiv preprint doi: https://doi.org/10.1101/2021.04.21.440862; this version posted April 22, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Rapid induction of antigen-specific CD4+ T cells guides coordinated humoral and cellular immune responses to SARS-CoV-2 mRNA vaccination Authors: Mark M. Painter1,2, †, Divij Mathew1,2, †, Rishi R. Goel1,2, †, Sokratis A. Apostolidis1,2,3, †, Ajinkya Pattekar2, Oliva Kuthuru1, Amy E. Baxter1, Ramin S. Herati4, Derek A. Oldridge1,5, Sigrid Gouma6, Philip Hicks6, Sarah Dysinger6, Kendall A. Lundgreen6, Leticia Kuri-Cervantes1,6, Sharon Adamski2, Amanda Hicks2, Scott Korte2, Josephine R. Giles1,7,8, Madison E. Weirick6, Christopher M. McAllister6, Jeanette Dougherty1, Sherea Long1, Kurt D’Andrea1, Jacob T. Hamilton2,6, Michael R. Betts1,6, Paul Bates6, Scott E. Hensley6, Alba Grifoni9, Daniela Weiskopf9, Alessandro Sette9, Allison R. Greenplate1,2, E. John Wherry1,2,7,8,* Affiliations 1 Institute for Immunology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA 2 Immune Health™, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA 3 Division of Rheumatology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA 4 NYU Langone Vaccine Center, Department of Medicine, New York University School of Medicine, New York, NY 5 Department -

Lymphocyte-Activation Gene 3 (LAG-3) Immune Pathway
Lymphocyte-Activation Gene 3 (LAG-3) About LAG-3 LAG-3 Lymphocyte-activation gene 3 (LAG-3) is an immune checkpoint receptor protein found on the cell surface of effector T cells and regulatory T cells (Tregs) and functions to control T cell response, activation and growth.1 TCR T cells are a type of white blood cell that are part of the immune system. Activation of cytotoxic T cells by antigens enables them to 1 kill unhealthy or foreign cells. Inactive T cell Antigen MHC Dendritic cell (APC) LAG-3 and LAG-3 and LAG-3 and Immune Function T Cell Exhaustion Cancer • After a T cell is activated to kill its • However, in certain situations where T • Because of its critical role in regulating target cell, LAG-3 expression is cells experience prolonged exposure to an exhaustion of cytotoxic T cells and Treg increased to turn off the immune antigen, such as cancer or chronic function, LAG-3 has become a target of response, so that the T cell does not go infection, the T cells become desensitized study in the cancer field. on to attack healthy cells.2 and lose their ability to activate and multiply in the presence of the antigen.4 • In cancer, LAG-3 expressing exhausted • Inhibition of the immune response is cytotoxic T cells and Tregs expressing accomplished through activation of • The desensitized T cell will also LAG-3 gather at tumor sites.5,6 the LAG-3 pathway, which can occur progressively fail to produce cytokines via binding of LAG-3 to a type of (proteins that assist in the immune • Preclinical studies suggest that inhibiting antigen-presenting complex called response) and kill the target cells.4 LAG-3 allows T cells to regain their MHC II. -

Understanding the Immune System: How It Works
Understanding the Immune System How It Works U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH National Institute of Allergy and Infectious Diseases National Cancer Institute Understanding the Immune System How It Works U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH National Institute of Allergy and Infectious Diseases National Cancer Institute NIH Publication No. 03-5423 September 2003 www.niaid.nih.gov www.nci.nih.gov Contents 1 Introduction 2 Self and Nonself 3 The Structure of the Immune System 7 Immune Cells and Their Products 19 Mounting an Immune Response 24 Immunity: Natural and Acquired 28 Disorders of the Immune System 34 Immunology and Transplants 36 Immunity and Cancer 39 The Immune System and the Nervous System 40 Frontiers in Immunology 45 Summary 47 Glossary Introduction he immune system is a network of Tcells, tissues*, and organs that work together to defend the body against attacks by “foreign” invaders. These are primarily microbes (germs)—tiny, infection-causing Bacteria: organisms such as bacteria, viruses, streptococci parasites, and fungi. Because the human body provides an ideal environment for many microbes, they try to break in. It is the immune system’s job to keep them out or, failing that, to seek out and destroy them. Virus: When the immune system hits the wrong herpes virus target or is crippled, however, it can unleash a torrent of diseases, including allergy, arthritis, or AIDS. The immune system is amazingly complex. It can recognize and remember millions of Parasite: different enemies, and it can produce schistosome secretions and cells to match up with and wipe out each one of them. -

Immunology 101
Immunology 101 Justin Kline, M.D. Assistant Professor of Medicine Section of Hematology/Oncology Committee on Immunology University of Chicago Medicine Disclosures • I served as a consultant on Advisory Boards for Merck and Seattle Genetics. • I will discuss non-FDA-approved therapies for cancer 2 Outline • Innate and adaptive immune systems – brief intro • How immune responses against cancer are generated • Cancer antigens in the era of cancer exome sequencing • Dendritic cells • T cells • Cancer immune evasion • Cancer immunotherapies – brief intro 3 The immune system • Evolved to provide protection against invasive pathogens • Consists of a variety of cells and proteins whose purpose is to generate immune responses against micro-organisms • The immune system is “educated” to attack foreign invaders, but at the same time, leave healthy, self-tissues unharmed • The immune system can sometimes recognize and kill cancer cells • 2 main branches • Innate immune system – Initial responders • Adaptive immune system – Tailored attack 4 The immune system – a division of labor Innate immune system • Initial recognition of non-self (i.e. infection, cancer) • Comprised of cells (granulocytes, monocytes, dendritic cells and NK cells) and proteins (complement) • Recognizes non-self via receptors that “see” microbial structures (cell wall components, DNA, RNA) • Pattern recognition receptors (PRRs) • Necessary for priming adaptive immune responses 5 The immune system – a division of labor Adaptive immune system • Provides nearly unlimited diversity of receptors to protect the host from infection • B cells and T cells • Have unique receptors generated during development • B cells produce antibodies which help fight infection • T cells patrol for infected or cancerous cells • Recognize “foreign” or abnormal proteins on the cell surface • 100,000,000 unique T cells are present in all of us • Retains “memory” against infections and in some cases, cancer. -

Early Lymphocyte Recovery Predicts Longer Survival After Autologous Peripheral Blood Stem Cell Transplantation in Multiple Myeloma
Bone Marrow Transplantation (2006) 37, 1037–1042 & 2006 Nature Publishing Group All rights reserved 0268-3369/06 $30.00 www.nature.com/bmt ORIGINAL ARTICLE Early lymphocyte recovery predicts longer survival after autologous peripheral blood stem cell transplantation in multiple myeloma H Kim1, H-J Sohn2, S Kim2, J-S Lee2, W-K Kim2 and C Suh2 1Division of Hematology-Oncology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea and 2Division of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea To understand the prognostic value of lymphocyte Although the use of allogeneic hematopoietic stem cell recovery after autologous peripheral blood stem cell transplantation has been increasing, autologous stem cell transplantation (APBSCT), we performed a retrospective transplantation (ASCT) remains the standard therapeutic study of 59 newly diagnosed multiple myeloma (MM) modality for MM.1–3 The main drawback of ASCT is its patients who underwent frontline APBSCT. Conditioning high relapse rate. Because immune-mediated tumor eradi- regimens were melphalan 100 mg/m2 for 2 days. Follow- cation may lower the relapse rate associated with ASCT, ing APBSCT, all patients showed complete or partial there is increasing interest in immune reconstitution response. Median follow-up time was 29.57 months and following ASCT.4–9 In support of this, a relationship median recovery of absolute lymphocyte count (ALC) between lymphocyte recovery after ASCT and relapse rate X1000/mm3 was 23 days. Univariate analysis revealed or survival has been observed in many diseases, suggesting that significant predictors of overall survival (OS) that early lymphocyte recovery associated with immune included bone marrow (BM) plasma cells p40% at reconstitution can act against residual disease progres- diagnosis (P ¼ 0.0243) and recovery of ALC X1000/mm3 sion.10–17 by day þ 23 (P ¼ 0.0156). -

The CLL Guide Information for Patients and Caregivers Chronic Lymphocytic Leukemia
The CLL Guide Information for Patients and Caregivers Chronic Lymphocytic Leukemia Laura, CLL survivor This publication was supported by Revised 2014 Inside Front Cover A Message from Louis J. DeGennaro, PhD President and CEO of The Leukemia & Lymphoma Society The Leukemia & Lymphoma Society (LLS) is the world’s largest voluntary health organization dedicated to finding cures for blood cancer patients. Since 1954, we have invested over $1 billion in research specifically targeting blood cancers to advance therapies and save lives. We will continue to invest in research for cures, programs and services to improve the quality of life for people with chronic lymphocytic leukemia (CLL). We know that understanding CLL can be tough. We are here to help and are committed to provide you with the most up-to-date information about CLL, your treatment and your support options. We know how important it is for you to understand your health information and to use it with your healthcare team toward good health, remission and recovery. Our vision is that one day all people with CLL will be cured or be able to manage their disease with good quality of life. Until then, we trust the information in this Guide will help you along your journey. We wish you well. Louis J. DeGennaro, PhD President and Chief Executive Officer The Leukemia & Lymphoma Society Inside This Guide 2 Introduction 3 Resources and Information 7 Part 1—Understanding CLL About Blood The Immune System What is CLL? Signs and Symptoms Diagnosing CLL Tracking Your CLL Tests 14 Part 2—Treating CLL Finding the Right Doctor Treatment Planning Treatments for CLL Treatment for Relapsed or Refractory CLL Stem Cell Transplantation 26 Part 3—About Clinical Trials 27 Part 4—Side Effects and Treatment Response Side Effects of CLL Treatment Treatment Response 29 Follow-up Care 30 Ongoing Care 31 Health Terms 34 Healthcare Question Guides This LLS guide about CLL is for information only. -

Vaccine Immunology Claire-Anne Siegrist
2 Vaccine Immunology Claire-Anne Siegrist To generate vaccine-mediated protection is a complex chal- non–antigen-specifc responses possibly leading to allergy, lenge. Currently available vaccines have largely been devel- autoimmunity, or even premature death—are being raised. oped empirically, with little or no understanding of how they Certain “off-targets effects” of vaccines have also been recog- activate the immune system. Their early protective effcacy is nized and call for studies to quantify their impact and identify primarily conferred by the induction of antigen-specifc anti- the mechanisms at play. The objective of this chapter is to bodies (Box 2.1). However, there is more to antibody- extract from the complex and rapidly evolving feld of immu- mediated protection than the peak of vaccine-induced nology the main concepts that are useful to better address antibody titers. The quality of such antibodies (e.g., their these important questions. avidity, specifcity, or neutralizing capacity) has been identi- fed as a determining factor in effcacy. Long-term protection HOW DO VACCINES MEDIATE PROTECTION? requires the persistence of vaccine antibodies above protective thresholds and/or the maintenance of immune memory cells Vaccines protect by inducing effector mechanisms (cells or capable of rapid and effective reactivation with subsequent molecules) capable of rapidly controlling replicating patho- microbial exposure. The determinants of immune memory gens or inactivating their toxic components. Vaccine-induced induction, as well as the relative contribution of persisting immune effectors (Table 2.1) are essentially antibodies— antibodies and of immune memory to protection against spe- produced by B lymphocytes—capable of binding specifcally cifc diseases, are essential parameters of long-term vaccine to a toxin or a pathogen.2 Other potential effectors are cyto- effcacy.