ANGIOSPERM CLASSIFICATION 1. DICOTYLEDONS – 2 Cotyledons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introduction: the Tiliaceae and Genustilia
Cambridge University Press 978-0-521-84054-5 — Lime-trees and Basswoods Donald Pigott Excerpt More Information Introduction: the 1 Tiliaceae and genus Tilia Tilia is the type genus of the family name Tiliaceae Juss. (1789), The ovary is syncarpous with five or more carpels but only and T. × europaea L.thetypeofthegenericname(Jarviset al. one style and a stigma with a lobe above each carpel. In Tili- 1993). Members of Tiliaceae have many morphological char- aceae, the ovules are anatropous. In Malvaceae, filaments of acters in common with those of Malvaceae Juss. (1789) and the stamens are fused into a tube but have separate apices that both families were placed in the order Malvales by Engler each bear a unilocular anther. Staminodes are absent. Each of (1912). In Engler’s treatment, Tiliaceae consisted mainly of five or more carpels supports a separate style, which together trees and shrubs belonging to several genera, including a pass through the staminal tube so that the stigmas are exposed few herbaceous genera, almost all occurring in the warmer above the anthers. The ovules may be either anatropous or regions. campylotropous. This treatment was revised by Engler and Diels (1936). The Molecular studies comprising sequence analysis of DNA of family was retained by Cronquist (1981) and consisted of about two plastid genes (Bayer et al. 1999) show that, in general, the 50 genera and 700 species distributed in the tropics and warmer inclusion of most genera, including Tilia, traditionally placed parts of the temperate zones in Asia, Africa, southern Europe in Malvales is correct. There is, however, clear evidence that and America. -

Alien Flora of Europe: Species Diversity, Temporal Trends, Geographical Patterns and Research Needs
Preslia 80: 101–149, 2008 101 Alien flora of Europe: species diversity, temporal trends, geographical patterns and research needs Zavlečená flóra Evropy: druhová diverzita, časové trendy, zákonitosti geografického rozšíření a oblasti budoucího výzkumu Philip W. L a m b d o n1,2#, Petr P y š e k3,4*, Corina B a s n o u5, Martin H e j d a3,4, Margari- taArianoutsou6, Franz E s s l7, Vojtěch J a r o š í k4,3, Jan P e r g l3, Marten W i n t e r8, Paulina A n a s t a s i u9, Pavlos A n d r i opoulos6, Ioannis B a z o s6, Giuseppe Brundu10, Laura C e l e s t i - G r a p o w11, Philippe C h a s s o t12, Pinelopi D e l i p e t - rou13, Melanie J o s e f s s o n14, Salit K a r k15, Stefan K l o t z8, Yannis K o k k o r i s6, Ingolf K ü h n8, Hélia M a r c h a n t e16, Irena P e r g l o v á3, Joan P i n o5, Montserrat Vilà17, Andreas Z i k o s6, David R o y1 & Philip E. H u l m e18 1Centre for Ecology and Hydrology, Hill of Brathens, Banchory, Aberdeenshire AB31 4BW, Scotland, e-mail; [email protected], [email protected]; 2Kew Herbarium, Royal Botanic Gardens Kew, Richmond, Surrey, TW9 3AB, United Kingdom; 3Institute of Bot- any, Academy of Sciences of the Czech Republic, CZ-252 43 Průhonice, Czech Republic, e-mail: [email protected], [email protected], [email protected], [email protected]; 4Department of Ecology, Faculty of Science, Charles University, Viničná 7, CZ-128 01 Praha 2, Czech Republic; e-mail: [email protected]; 5Center for Ecological Research and Forestry Applications, Universitat Autònoma de Barcelona, 08193 Bellaterra, Spain, e-mail: [email protected], [email protected]; 6University of Athens, Faculty of Biology, Department of Ecology & Systematics, 15784 Athens, Greece, e-mail: [email protected], [email protected], [email protected], [email protected], [email protected]; 7Federal Environment Agency, Department of Nature Conservation, Spittelauer Lände 5, 1090 Vienna, Austria, e-mail: [email protected]; 8Helmholtz Centre for Environmental Research – UFZ, Department of Community Ecology, Theodor-Lieser- Str. -

Downloaded from Brill.Com10/07/2021 08:53:11AM Via Free Access 130 IAWA Journal, Vol
IAWA Journal, Vol. 27 (2), 2006: 129–136 WOOD ANATOMY OF CRAIGIA (MALVALES) FROM SOUTHEASTERN YUNNAN, CHINA Steven R. Manchester1, Zhiduan Chen2 and Zhekun Zhou3 SUMMARY Wood anatomy of Craigia W.W. Sm. & W.E. Evans (Malvaceae s.l.), a tree endemic to China and Vietnam, is described in order to provide new characters for assessing its affinities relative to other malvalean genera. Craigia has very low-density wood, with abundant diffuse-in-aggre- gate axial parenchyma and tile cells of the Pterospermum type in the multiseriate rays. Although Craigia is distinct from Tilia by the pres- ence of tile cells, they share the feature of helically thickened vessels – supportive of the sister group status suggested for these two genera by other morphological characters and preliminary molecular data. Although Craigia is well represented in the fossil record based on fruits, we were unable to locate fossil woods corresponding in anatomy to that of the extant genus. Key words: Craigia, Tilia, Malvaceae, wood anatomy, tile cells. INTRODUCTION The genus Craigia is endemic to eastern Asia today, with two species in southern China, one of which also extends into northern Vietnam and southeastern Tibet. The genus was initially placed in Sterculiaceae (Smith & Evans 1921; Hsue 1975), then Tiliaceae (Ren 1989; Ying et al. 1993), and more recently in the broadly circumscribed Malvaceae s.l. (including Sterculiaceae, Tiliaceae, and Bombacaceae) (Judd & Manchester 1997; Alverson et al. 1999; Kubitzki & Bayer 2003). Similarities in pollen morphology and staminodes (Judd & Manchester 1997), and chloroplast gene sequence data (Alverson et al. 1999) have suggested a sister relationship to Tilia. -

Microcos Antidesmifolia (Malvaceae-Grewioideae), a Poorly Known Species in Singapore
Gardens' Bulletin Singapore 72(2): 159–164. 2020 159 doi: 10.26492/gbs72(2).2020-04 Microcos antidesmifolia (Malvaceae-Grewioideae), a poorly known species in Singapore S.K. Ganesan1, R.C.J. Lim2, P.K.F. Leong1 & X.Y. Ng2 1Singapore Botanic Gardens, National Parks Board, 1 Cluny Road, 259569 Singapore [email protected] 2 Native Plant Centre, Horticulture and Community Gardening Division, National Parks Board, 100K Pasir Panjang Road, 118526 Singapore ABSTRACT. A poorly known species in Singapore, Microcos antidesmifolia (King) Burret, is described and illustrated for the first time. In Singapore, it is known from the type variety, Microcos antidesmifolia (King) Burret var. antidesmifolia. Notes on distribution, ecology and conservation status are given. This species is assessed as Critically Endangered for Singapore. A key is given for the fiveMicrocos L. species in Singapore. Keywords. Conservation assessment, distribution, ecology, flora Introduction The genus Microcos L. comprises about 80 species that are distributed in tropical Africa (not in Madagascar), India, Sri Lanka, Myanmar, Indochina, south China and throughout Malesia (except the Lesser Sunda Islands) (Chung & Soepadmo, 2011). Until about 2007, Microcos was placed in the family Tiliaceae. However, phylogenetic analysis using both molecular and morphological data has led to the recognition of an expanded Malvaceae, composed of the formerly recognised families Malvaceae s.s., Tiliaceae, Bombacaceae and Sterculiaceae, and for the Malvaceae s.l. to be divided into nine sub-families (Alverson et al., 1999; Bayer et al., 1999; Bayer & Kubitzki, 2003). This classification was adopted by the Angiosperm Phylogeny Group (APG, 2009, 2016). Here we follow APG and consider Microcos in Malvaceae, subfamily Grewioideae Dippel. -

The Fossil Record of Angiosperm Families in Relation to Baraminology
The Proceedings of the International Conference on Creationism Volume 7 Article 31 2013 The Fossil Record of Angiosperm Families in Relation to Baraminology Roger W. Sanders Bryan College Follow this and additional works at: https://digitalcommons.cedarville.edu/icc_proceedings DigitalCommons@Cedarville provides a publication platform for fully open access journals, which means that all articles are available on the Internet to all users immediately upon publication. However, the opinions and sentiments expressed by the authors of articles published in our journals do not necessarily indicate the endorsement or reflect the views of DigitalCommons@Cedarville, the Centennial Library, or Cedarville University and its employees. The authors are solely responsible for the content of their work. Please address questions to [email protected]. Browse the contents of this volume of The Proceedings of the International Conference on Creationism. Recommended Citation Sanders, Roger W. (2013) "The Fossil Record of Angiosperm Families in Relation to Baraminology," The Proceedings of the International Conference on Creationism: Vol. 7 , Article 31. Available at: https://digitalcommons.cedarville.edu/icc_proceedings/vol7/iss1/31 Proceedings of the Seventh International Conference on Creationism. Pittsburgh, PA: Creation Science Fellowship THE FOSSIL RECORD OF ANGIOSPERM FAMILIES IN RELATION TO BARAMINOLOGY Roger W. Sanders, Ph.D., Bryan College #7802, 721 Bryan Drive, Dayton, TN 37321 USA KEYWORDS: Angiosperms, flowering plants, fossils, baramins, Flood, post-Flood continuity criterion, continuous fossil record ABSTRACT To help estimate the number and boundaries of created kinds (i.e., baramins) of flowering plants, the fossil record has been analyzed. To designate the status of baramin, a criterion is applied that tests whether some but not all of a group’s hierarchically immediate subgroups have a fossil record back to the Flood (accepted here as near the Cretaceous-Paleogene boundary). -

Appendix 1 Vernacular Names
Appendix 1 Vernacular Names The vernacular names listed below have been collected from the literature. Few have phonetic spellings. Spelling is not helped by the difficulties of transcribing unwritten languages into European syllables and Roman script. Some languages have several names for the same species. Further complications arise from the various dialects and corruptions within a language, and use of names borrowed from other languages. Where the people are bilingual the person recording the name may fail to check which language it comes from. For example, in northern Sahel where Arabic is the lingua franca, the recorded names, supposedly Arabic, include a number from local languages. Sometimes the same name may be used for several species. For example, kiri is the Susu name for both Adansonia digitata and Drypetes afzelii. There is nothing unusual about such complications. For example, Grigson (1955) cites 52 English synonyms for the common dandelion (Taraxacum officinale) in the British Isles, and also mentions several examples of the same vernacular name applying to different species. Even Theophrastus in c. 300 BC complained that there were three plants called strykhnos, which were edible, soporific or hallucinogenic (Hort 1916). Languages and history are linked and it is hoped that understanding how lan- guages spread will lead to the discovery of the historical origins of some of the vernacular names for the baobab. The classification followed here is that of Gordon (2005) updated and edited by Blench (2005, personal communication). Alternative family names are shown in square brackets, dialects in parenthesis. Superscript Arabic numbers refer to references to the vernacular names; Roman numbers refer to further information in Section 4. -

Bentham and Hooker Classification Faculty Name - Dr Piyush Kumar Rai Email – [email protected]
Course - B.Sc. Botany Semester - II Paper Code - BOT GE202 Paper Name – Plant Ecology and Taxonomy Topic - Bentham and Hooker Classification Faculty Name - Dr Piyush Kumar Rai Email – [email protected] Bentham and Hooker Classification George Bentham (1800 – 1884) Joseph Hooker (1817 – 1911) It was Proposed by George Bentham ( 1800 – 1884 ) and Joseph Dalton Hooker (1817 – 1911 ) in their Genera Plantarum published during July (1862 ) & April ( 1883 ) George Bentham (1800 – 1884) and Joseph Dalton Hooker ( 1817 ) – 1911) , the two British botanist who were associated with the Royal Botanic garden at kew ( England) gave most important and easily workable system of classification of angiosperms and published it in three volume of ‘Genera plantarum ‘ The first part of Genera plantarum appeared in July 1882 and the last part in April 1883 . This was the greatest taxonomic work ever produced in the united kingdom and ever since been an inspiration to generations of kew botanists . Although Bentham and Hooker’s system of classification was based on that of A.P. de Candolle but greater stress was given on contrast between free and fused petals . Their symptom was widely accepted in Britain and commonwealth countries but in Europe and North America it did not hold the much ground . Bentham and Hooker divided the seed plants into Dicotyledons, Gymnosperms and Monocotyledons. They placed Ranales in beginning and grasses at the end . The following is the summary of Bentham & Hooker’s system. DICOTYLEDONS : A . Polypetalae ( petals are free ) Series l Thalamiflorae Order 1. Ranales eg. Ranunculaceae, Magnollaceae e.t.c Order 2. Parietales eg. Papaveraceae , Cruciferae e.t.c Order 3. -

POLYPETALAE – Petals Separate THALAMIFLORAE – Sepals, Petals and Stamens All Attached to Receptacle
POLYPETALAE – petals separate THALAMIFLORAE – Sepals, Petals and Stamens all attached to receptacle. Gynoecium apocarpous. RANUNCULACEAE (Herbaceous, leaves 3-parted) BERBERIDACEAE* (Carpel solitary, Anthers with flaps). Parietal placentation. [NOT Natural. Convergent evolution: Papaveraceae close to Ranunc., but remainder scattered amongst Rosids] PAPAVERACEAE* (Sepals 2, petals 4) CRUCIFERAE* (Petals 4, Stamens 6, ovary 2) CAPPARACEAE* (Ovary stalked) RESEDACEAE (Ovary open, 3-parted) CISTACEAE VIOLACEAE Ovary 2-3 septate. PITTOSPORACEAE* POLYGALACEAE. Axile placentation. CARYOPHYLLACEAE* PORTULACACEAE (Sepals gland-fringed) Stamens numerous; Calyx imbricate. GUTTIFERAE/CLUSIACEAE* THEACEAE Stamens numerous; Calyx valvate. MALVACEAE* (Anthers 1-celled) STERCULIACEAE TILIACEAE. DISCIFLORAE – Ovary superior, immersed in disk of flower. Ovule pendulous, raphe ventral;multiple series of stamens. LINACEAE; GERANIACEAE*; RUTACEAE Ovule pendulous, raphe dorsal OLACACEAE; AQUIFOLIACEAE*. Ovule erect, raphe ventral CELASTRACEAE; RHAMNACEAE*; VITACEAE. Ovule ascending, raphe ventral to reversed SAPINDACEAE*; ANACARDIACEAE CALYCIFLORAE – Stamens fused to Calyx of flower Ovaries separate, rarely united LEGUMINOSAE ROSACEAE* [SAXIFRAGALES. Carpels ±fused, separate styles: SAXIFRAGACEAE* (2 carps) CRASSULACEAE (5-6 carps) HAMAMELIDACEAE (2)] [: HYDRANGEACEAE – opp leaves, syncarpous. ESCALLONIACEAE – alt leaves dry pod => ASTERIDS Ovary syncarpous; divided into locules. MYRTACEAE (stamens numerous) LYTHRACEAE* ONAGRACEAE*. Ovary syncarpous; Parietal placentation LOASACEAE; TURNERACEAE; PASSIFLORACEAE; CUCURBITACEAE*; BEGONIACEAE; DATISCACEAE. Ficoidales – Ovary syncarpous; sub-basal placentation [the basal placentation is critical in placing these families among the Caryophyllids see above] CACTACEAE; AIZOACEAE.] Umbellales – Ovary syncarpous; 1 ovule per locule. [these families belong amongst the basal Asterids. Inferior ovaries but with separate petals] UMBELLIFERAE (2-locules); ARALIACEAE (5-locules); CORNACEAE. . -
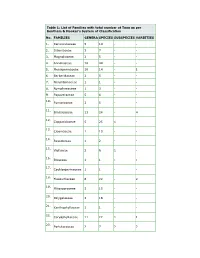
Table 1: List of Families with Total Number of Taxa As Per Bentham & Hooker’S System of Classification
Table 1: List of Families with total number of Taxa as per Bentham & Hooker’s System of Classification No. FAMILIES GENERA SPECIES SUBSPECIES VARIETIES 1. Ranunculaceae 5 14 - - 2. Dilleniaceae 3 7 - - 3. Magnoliaceae 2 5 - - 4. Annonaceae 16 44 - - 5. Menispermaceae 10 14 - 1 6. Berberidaceae 2 5 - - 7. Nelumbonaceae 1 1 - - 8. Nymphaeaceae 1 3 - - 9. Papaveraceae 5 6 - - 10. Fumariaceae 3 5 - - 11. Brassicaceae 13 24 - 4 12. Capparidaceae 5 25 1 - 13. Cleomaceae 1 10 - - 14. Resedaceae 1 2 - - 15. Violaceae 2 6 1 - 16. Bixaceae 1 1 - - 17. Cochlospermaceae 1 1 - - 18. Flacourtiaceae 8 22 - 2 19. Pittosporaceae 3 15 - - 20. Polygalaceae 3 18 - - 21. Xanthophyllaceae 1 1 - - 22. Caryophyllaceae 11 22 1 1 23. Portulacaceae 2 7 2 2 24. Tamaricaceae 1 3 - - 25. Elatinaceae 2 5 - - 26. Hypericaceae 1 7 - - 27. Clusiaceae 4 17 2 2 28. Bonnetiaceae 1 2 - - 29. Theaceae 5 6 - - 30. Actinidiaceae 1 2 - - 31. Stachyuraceae 1 1 - - 32. Dipterocarpaceae 4 9 - - 33. Ancistrocladaceae 1 1 - - 34. Malvaceae 18 64 4 - 35. Bombacaceae 5 6 - - 36. Sterculiaceae 19 43 - - 37. Tiliaceae 5 37 - - 38. Elaeocarpaceae 2 9 - - 39. Linaceae 3 4 - - 40. Erythroxylaceae 1 5 - - 41. Malpighiaceae 5 10 - - 42. Zygophyllaceae 3 5 - - 43. Geraniaceae 3 9 - - 44. Tropaeolaceae 1 2 - - 45. Limnanthaceae 1 1 - - 46. Oxalidaceae 2 17 - 6 47. Averrhoaceae 1 2 - - 48. Balsaminaceae 3 63 - 2 49. Rutaceae 23 53 - 2 50. Simaroubaceae 2 3 - - 51. Surianaceae 1 1 - - 52. Balanitaceae 1 1 - - 53. Ochnaceae 2 4 - 2 54. Burseraceae 5 9 - 1 55. Meliaceae 18 31 - 8 56. -

Phytosociological Study of Coastal Flora of Devbhoomi Dwarka District and Its Islands in the Gulf of Kachchh, Gujarat
International Journal of Scientific Research in _______________________________ Research Paper . Biological Sciences Vol.6, Issue.3, pp.01-13, June (2019) E-ISSN: 2347-7520 DOI: https://doi.org/10.26438/ijsrbs/v6i3.113 Phytosociological study of coastal flora of Devbhoomi Dwarka district and its islands in the Gulf of Kachchh, Gujarat L. Das1*, H. Salvi2, R. D. Kamboj 3 1,3Gujarat Ecological Education and Research Foundation, Gandhinagar, Gujarat, India 2Department of Botany, Songadh Government Science College, Tapi, Gujarat, India *Corresponding Author: [email protected]; Tel.: +91-7573020436 Available online at: www.isroset.org Received: 16/May/2019, Accepted: 02/Jun/2019, Online: 30/Jun/2019 Abstract- The study described the diversity and phytosociological attributes of plant species (trees, shrubs and herbs) in coastal areas of Devbhoomi Dwarka District and its islands in the Gulf of Kachchh. A random sampling method was employed in this study. A total of 243 plant species were recorded of which trees and shrubs represented with 30 specieseach. Grasses & sedges were also represented by 30 species and 29 species were climbers. Among the tree and shrub species, Prosopis juliflora showed the highest density (373.51 ind. /ha), frequency (63.50.67%), relative density (30.19.7%), relative frequency (24.41%) and relative abundance (7.68%).Regarding herb species, Aristida redacta represented the highest density (3.97ind./sq.m) and frequency (39.02%). Moreover, the highest importance value index was measured in Prosopis juliflora (62.28) among trees & shrubs and Aristida redacta (31.51) among herbs. The Abundance/Frequency ratio of trees, shrubs and herb species showed contagious distribution pattern within the study area. -

Systematic Anatomy of the Woods of the Tiliaceae
Technical Bulletin 158 June 1943 Systematic Anatomy of the Woods of the Tiliaceae B. Francis Kukachka and L. W. Rees Division of Forestry University of Minnesota Agricultural Experiment Station Systematic Anatomy of the Woods of the Tiliaceae B. Francis Kukachka and L. W. Rees Division of Forestry University of Minnesota Agricultural Experiment Station Accepted for publication January 29, 1943 CONTENTS Page Introduction 3 Anatomical indicators of phylogeny 4 Taxonomic history 7 Materials and methods 12 Measurements 14 Vessel members 14 Pore diameter 15 Numerical distributionS of pores 15 Pore grouping 15 Pore wall thickness 15 Fiber length 16 Fiber diameter 16 Parenchyma width and length 16 Description of the woods of the Tiliaceae 16 Description of the woods of the Elaeocarpaceae 49 Discussion 54 Elaeocarpaceae 54 Tiliaceae 56 General conclusions 63 Summary 64 Acknowledgments 65 Literature cited 65 2M-6-43 Systematic Anatomy of the Woods of the Tiliaceae B. Francis Kukachka and L. W. Rees INTRODUCTION ITHIN the last 20 years there has been developed a method Wof studying evolutionary trends in the secondary xylem of the dicotyledons, the fundamentals of which were laid principally by the researches of Bailey and Tupper( 13), Frost (50, 51, 52), and Kribs (64, 65). The technique depends on the previous establishment of an undoubtedly primitive anatomical feature and this is then asso- ciated with the feature to be investigated in order to determine the extent and direction of the correlation between the occur- rence of both features in the various species. A high positive correlation would indicate that the feature studied is relatively primitive. -

Bentham and Hooker's Classification (1862 – ’83)
Bentham and Hooker's classification (1862 – ’83) George Bentham and Joseph Dalton Hooker - Two English taxonomists who were closely associated with the Royal Botanical Garden at Kew, England have given a detailed classification of plant kingdom, particularly the angiosperms. They gave an outstanding system of classification of phanerogams in their Genera Plantarum which was published in three volumes between the years 1862 to 1883. It is a natural system of classification. However, it does not show the evolutionary relationship between different groups of plants, in the strict sense. Nevertheless, it is the most popular system of classification particularly for angiosperms. The popularity comes from the face that very clear key characters have been listed for each of the families. These key characters enable the students of taxonomy to easily identify and assign any angiosperm plant to its family. Bentham and Hooker have grouped advanced, seed bearing plants into a major division called Phanerogamia. This division has been divided into three classes namely: 1. Dicotyledonae 2. Gymnospermae and 3. Monocotyledoneae 84 45 36 3 34 PhanerogamsSummaryor spermatophyta of Benthamare anddivided Hooker'sinto three classificationclasses - Dicotyledonae (1862 – 83), Gymnospermae and Monocotyledonae Class - Dicotyledonae– two cotyledons, open vascular bundles, reticulate venation I. Sub-Class Polypetalae - The flowers are usually with two distinct whorls of perianth; the segments of the inner whorl or "corolla" are free. A. Series-Thalamiflorae -(The calyx consists of usually distinct sepals, which are free from the ovary; doom shaped thalamus). 6 Orders/Cohort; 34 Families or Natural orders -R B. Series – Disciflorae - The calyx consists of either distinct or united sepals, which may be free or adnate to the ovary; a prominent ring of cushion shaped disc is usually present below the ovary, sometimes broken up into glands; the stamens are usually definite in number, inserted upon, or at the outer or inner base of the disc; the ovary is superior.