Response of Coccinellid Community to the Dimethoate Application in Olive Groves in Northeastern Portugal S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coccinellidae)
ECOLOGY AND BEHAVIOUR OF THE LADYBIRD BEETLES (COCCINELLIDAE) Edited by I. Hodek, H.E van Emden and A. Honek ©WILEY-BLACKWELL A John Wiley & Sons, Ltd., Publication CONTENTS Detailed contents, ix 8. NATURAL ENEMIES OF LADYBIRD BEETLES, 375 Contributors, xvii Piotr Ccryngier. Helen E. Roy and Remy L. Poland Preface, xviii 9. COCCINELLIDS AND [ntroduction, xix SEMIOCHEMICALS, 444 ]an Pettcrsson Taxonomic glossary, xx 10. QUANTIFYING THE IMPACT OF 1. PHYLOGENY AND CLASSIFICATION, 1 COCCINELLIDS ON THEIR PREY, 465 Oldrich Nedved and Ivo Kovdf /. P. Mid'laud and James D. Harwood 2. GENETIC STUDIES, 13 11. COCCINELLIDS IN BIOLOGICAL John J. Sloggett and Alois Honek CONTROL, 488 /. P. Midland 3. LIFE HISTORY AND DEVELOPMENT, 54 12. RECENT PROGRESS AND POSSIBLE Oldrkli Nedved and Alois Honek FUTURE TRENDS IN THE STUDY OF COCCINELLIDAE, 520 4. DISTRIBUTION AND HABITATS, 110 Helmut /; van Emden and Ivo Hodek Alois Honek Appendix: List of Genera in Tribes and Subfamilies, 526 5. FOOD RELATIONSHIPS, 141 Ivo Hodek and Edward W. Evans Oldrich Nedved and Ivo Kovdf Subject index. 532 6. DIAPAUSE/DORMANCY, 275 Ivo Hodek Colour plate pages fall between pp. 250 and pp. 251 7. INTRAGUILD INTERACTIONS, 343 Eric Lucas VII DETAILED CONTENTS Contributors, xvii 1.4.9 Coccidulinae. 8 1.4.10 Scymninae. 9 Preface, xviii 1.5 Future Perspectives, 10 References. 10 Introduction, xix Taxonomic glossary, xx 2. GENETIC STUDIES, 13 John J. Sloggett and Alois Honek 1. PHYLOGENY AND CLASSIFICATION, 1 2.1 Introduction, 14 Oldrich Nedved and Ivo Kovdf 2.2 Genome Size. 14 1.1 Position of the Family. 2 2.3 Chromosomes and Cytology. -

Phylogeny of Ladybirds (Coleoptera: Coccinellidae): Are the Subfamilies Monophyletic?
Molecular Phylogenetics and Evolution 54 (2010) 833–848 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Phylogeny of ladybirds (Coleoptera: Coccinellidae): Are the subfamilies monophyletic? A. Magro a,b,1, E. Lecompte b,c,*,1, F. Magné b,c, J.-L. Hemptinne a,b, B. Crouau-Roy b,c a Université de Toulouse, ENFA, EDB (Laboratoire Evolution et Diversité Biologique), 2 route de Narbonne, F-31320 Castanet Tolosan, France b CNRS, EDB (Laboratoire Evolution et Diversité Biologique), F-31062 Toulouse, France c Université de Toulouse, UPS, EDB (Laboratoire Evolution et Diversité Biologique), 118 route de Narbonne, F-31062 Toulouse, France article info abstract Article history: The Coccinellidae (ladybirds) is a highly speciose family of the Coleoptera. Ladybirds are well known Received 20 April 2009 because of their use as biocontrol agents, and are the subject of many ecological studies. However, little Revised 15 October 2009 is known about phylogenetic relationships of the Coccinellidae, and a precise evolutionary framework is Accepted 16 October 2009 needed for the family. This paper provides the first phylogenetic reconstruction of the relationships Available online 10 November 2009 within the Coccinellidae based on analysis of five genes: the 18S and 28S rRNA nuclear genes and the mitochondrial 12S, 16S rRNA and cytochrome oxidase subunit I (COI) genes. The phylogenetic relation- Keywords: ships of 67 terminal taxa, representative of all the subfamilies of the Coccinellidae (61 species, 37 genera), Phylogeny and relevant outgroups, were reconstructed using multiple approaches, including Bayesian inference Coccinellidae Partitioned analyses with partitioning strategies. The recovered phylogenies are congruent and show that the Coccinellinae Evolution is monophyletic but the Coccidulinae, Epilachninae, Scymninae and Chilocorinae are paraphyletic. -
Wikipedia Beetles Dung Beetles Are Beetles That Feed on Feces
Wikipedia beetles Dung beetles are beetles that feed on feces. Some species of dung beetles can bury dung times their own mass in one night. Many dung beetles, known as rollers , roll dung into round balls, which are used as a food source or breeding chambers. Others, known as tunnelers , bury the dung wherever they find it. A third group, the dwellers , neither roll nor burrow: they simply live in manure. They are often attracted by the dung collected by burrowing owls. There are dung beetle species of different colours and sizes, and some functional traits such as body mass or biomass and leg length can have high levels of variability. All the species belong to the superfamily Scarabaeoidea , most of them to the subfamilies Scarabaeinae and Aphodiinae of the family Scarabaeidae scarab beetles. As most species of Scarabaeinae feed exclusively on feces, that subfamily is often dubbed true dung beetles. There are dung-feeding beetles which belong to other families, such as the Geotrupidae the earth-boring dung beetle. The Scarabaeinae alone comprises more than 5, species. The nocturnal African dung beetle Scarabaeus satyrus is one of the few known non-vertebrate animals that navigate and orient themselves using the Milky Way. Dung beetles are not a single taxonomic group; dung feeding is found in a number of families of beetles, so the behaviour cannot be assumed to have evolved only once. Dung beetles live in many habitats , including desert, grasslands and savannas , [9] farmlands , and native and planted forests. They are found on all continents except Antarctica. They eat the dung of herbivores and omnivores , and prefer that produced by the latter. -

IOBC Internet Book of Biological Control – Draft September 2005
IOBC Internet Book of Biological Control Version 5, January 2008 IOBC Internet Book of Biological Control, version 5 Editor: J.C. van Lenteren ([email protected]) Aim: to present the history, the current state of affairs and the future of biological control in order to show that this control method is sound, safe and sustainable Contents 1. Introduction......................................................................................................................................................... 6 2. Discovery of natural enemies and a bit of entomological history ..................................................................... 10 3. Development of idea to use natural enemies for pest control and classification of types of biological control 16 4. History of biological control ............................................................................................................................. 22 5. Current situation of biological control (including region/country revieuws).................................................... 41 6. Biological control of weeds .............................................................................................................................. 51 7. Future of biological control: to be written ........................................................................................................ 61 8. Mass production, storage, shipment and release of natural enemies................................................................. 62 9. Commercial and non-commercial producers -
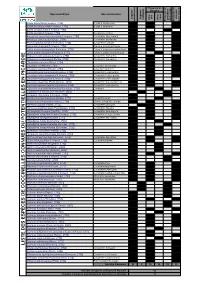
Liste Des Coccinelles De Picar
t s e n Présence en n a e e e d s o i s s s n i n e Picardie r e e e e e c g o i é u c c é c c l t é h q - è è n n n - r i h C e Nom scientifique Nom vernaculaire p t o e e p p n c u i e t a u l t s o r s s l s s l o o s q c x t n é i é é e i E E d e r r e a r u p A H d P P a d Adalia bipunctata (Linnaeus, 1758) Adalie à deux points 1 1 1 Adalia decempunctata (Linnaeus, 1758) Adalie à dix points 1 1 1 Adalia conglomerata (Linnaeus, 1758) 1 1 Anatis ocellata (Linnaeus, 1758) Coccinelle ocellée 1 1 1 Anisosticta novemdecimpunctata (Linnaeus, 1758) Coccinelle des roseaux 1 1 1 Aphidecta obliterata (Linnaeus, 1758) Coccinelle de l'épicea 1 1 1 Brumus quadripustulatus (Linnaeus, 1758) Coccinelle à virgule 1 1 1 Calvia decemguttata (Linnaeus, 1758) Calvia à dix points blancs 1 1 1 Calvia quatuordecimguttata (Linnaeus, 1758) Calvia à quatorze points blancs 1 1 1 Calvia quindecimguttata (Fabricius, 1777) Calvia à quinze points blancs 1 1 E Chilocorus bipustulatus (Linnaeus, 1758) Coccinelle des landes 1 1 1 I Chilocorus renipustulatus (Scriba, 1790) Coccinelle des saules 1 1 1 D Clitostethus arcuatus (Rossi, 1794) 1 1 1 R Coccidula rufa (Herbst, 1783) Coccidule des marais 1 1 1 A Coccidula scutellata (Herbst, 1783) Coccidule tachetée 1 1 1 C I Coccinella septempunctata (Linnaeus, 1758) Coccinelle à sept points 1 1 1 P Coccinella undecimpunctata (Linnaeus, 1758) Coccinelle à onze points 1 1 1 N Coccinella hieroglyphica (Linnaeus, 1758) Coccinelle à hiéroglyphe 1 1 E Coccinella magnifica (Redtenbacher, 1843) Coccinelle des fourmilières 1 1 1 Coccinella -

Biological Records Centre Report 1999-2004
CORE Metadata, citation and similar papers at core.ac.uk Provided by NERC Open Research Archive JNCC Report No. 370 BIOLOGICAL RECORDS CENTRE REPORT 1999-2004 This report should be cited as: Hill, M.O., Arnold, H.R., Broad, G.R., Burton, V.J., James, T.J., McLean, I.F.G., Preston, C.D., Rowland, F. & Roy, D.B. (2005) Biological Records Centre: Report 1999-2004 JNCC Report, No. 370 © JNCC, Peterborough 2005 Nominated Officers: Biological Records Centre Joint Nature Conservation Committee Dr Mark Hill Dr Ian McLean Biological Records Centre Joint Nature Conservation Committee CEH Monks Wood Monkstone House Abbots Ripton City Road Huntingdon PE28 2LS Peterborough PE1 1JY For further information please contact: Dr Ian McLean Joint Nature Conservation Committee Monkstone House, City Road Peterborough PE1 1JY ISSN 0963-8091 (Online) TABLE OF CONTENTS FOREWORD ........................................................................................................................................................1 INTRODUCTION ................................................................................................................................................2 ACKNOWLEDGEMENTS .................................................................................................................................3 PROGRAMME 1: DEVELOPING CAPACITY OF RECORDING SCHEMES AND VOLUNTEERS ....4 Developing National Schemes and Societies....................................................................................................4 PROGRAMME 2: DATA -
An Annotated Checklist of Ladybeetle Species (Coleoptera, Coccinellidae) of Portugal, Including the Azores and Madeira Archipelagos
ZooKeys 1053: 107–144 (2021) A peer-reviewed open-access journal doi: 10.3897/zookeys.1053.64268 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research An annotated checklist of ladybeetle species (Coleoptera, Coccinellidae) of Portugal, including the Azores and Madeira Archipelagos António Onofre Soares1, Hugo Renato Calado2, José Carlos Franco3, António Franquinho Aguiar4, Miguel M. Andrade5, Vera Zina3, Olga M.C.C. Ameixa6, Isabel Borges1, Alexandra Magro7,8 1 Centre for Ecology, Evolution and Environmental Changes and Azorean Biodiversity Group, Faculty of Sci- ences and Technology, University of the Azores, 9500-321, Ponta Delgada, Portugal 2 Azorean Biodiversity Group, Faculty of Sciences and Technology, University of the Azores, 9501-801, Ponta Delgada, Portugal 3 Centro de Estudos Florestais (CEF), Instituto Superior de Agronomia, Universidade de Lisboa, 1349-017, Lisboa, Portugal 4 Laboratório de Qualidade Agrícola, Caminho Municipal dos Caboucos, 61, 9135-372, Camacha, Madeira, Portugal 5 Rua das Virtudes, Barreiros Golden I, Bloco I, R/C B, 9000-645, Funchal, Madeira, Portugal 6 Centre for Environmental and Marine Studies and Department of Biology, University of Aveiro, Campus Universitário de Santiago, 3810-193, Aveiro, Portugal 7 Laboratoire Evolution et Diversité biologique, UMR 5174 CNRS, UPS, IRD, 118 rt de Narbonne Bt 4R1, 31062, Toulouse cedex 9, France 8 University of Toulouse – ENSFEA, 2 rt de Narbonne, Castanet-Tolosan, France Corresponding author: António Onofre Soares ([email protected]) Academic editor: J. Poorani | Received 10 February 2021 | Accepted 24 April 2021 | Published 2 August 2021 http://zoobank.org/79A20426-803E-47D6-A5F9-65C696A2E386 Citation: Soares AO, Calado HR, Franco JC, Aguiar AF, Andrade MM, Zina V, Ameixa OMCC, Borges I, Magro A (2021) An annotated checklist of ladybeetle species (Coleoptera, Coccinellidae) of Portugal, including the Azores and Madeira Archipelagos. -

Endemics Versus Newcomers: the Ladybird Beetle (Coleoptera: Coccinellidae) Fauna of Gran Canaria
insects Article Endemics Versus Newcomers: The Ladybird Beetle (Coleoptera: Coccinellidae) Fauna of Gran Canaria Jerzy Romanowski 1,* , Piotr Ceryngier 1 , Jaroslav Vetrovec˘ 2, Marta Piotrowska 3 and Karol Szawaryn 4 1 Institute of Biological Sciences, Cardinal Stefan Wyszy´nskiUniversity, Wóycickiego 1/3, 01-938 Warsaw, Poland; [email protected] 2 Buzulucká, 1105 Hradec Králové, Czech Republic; [email protected] 3 Faculty of Biology and Environmental Sciences UKSW, ul. Wóycickiego 1/3, PL-01-938 Warsaw, Poland; [email protected] 4 Museum and Institute of Zoology, Polish Academy of Sciences, Wilcza 64, 00-679 Warsaw, Poland; [email protected] * Correspondence: [email protected] Received: 18 August 2020; Accepted: 14 September 2020; Published: 18 September 2020 Simple Summary: Many plants and animals that live in the Canary Islands belong to the so-called endemic species, i.e., they do not occur outside of this particular region. Several other species have a slightly wider geographical distribution, apart from the Canaries, which also includes some islands of the nearby archipelagos, such as Madeira or the Azores, or the northwestern periphery of Africa. Here, we call such species subendemics. However, the Canary Islands have recently been colonized by a substantial number of immigrants from more or less remote areas. In this paper, based on our field survey and previously published data, we analyzed the fauna of the ladybird beetles (Coccinellidae) of Gran Canaria, one of the central islands of the archipelago. Among 42 ladybird beetle species so far recorded on this island, 17 (40%) are endemics and subendemics, and 21 (50%) probably arrived in Gran Canaria relatively recently, i.e., in the 20th and 21st century. -

Contribution to the Knowledge of the Coccinellidae of Sardinia (Coleoptera) *
ConservaZione habitat invertebrati 5: 501–516 (2011) Cnbfvr Contribution to the knowledge of the Coccinellidae of Sardinia ( Coleoptera)* Claudio CANEPARI Via Venezia 1, I-20097 San Donato Milanese (MI), Italy. E-mail: [email protected] *In: Nardi G., Whitmore D., Bardiani M., Birtele D., Mason F., Spada L. & Cerretti P. (eds), Biodiversity of Marganai and Montimannu (Sardinia). Research in the framework of the ICP Forests network. Conservazione Habitat Invertebrati, 5: 501–516. ABSTRACT An updated checklist of the Coccinellidae of Sardinia (Italy) is provided. Sixty-two species are recorded with certainty, while 19 other species (Scymnus (Mimopullus) fulvicollis (Mulsant, 1846), S. (M.) pharaonis Motschulsky, 1851, S. (Neopullus) haemorrhoidalis (Herbst, 1797), S. (N.) limbatus limbatus (Stephens, 1831), Nephus (Nephus) reunioni Fürsch, 1974, Scymniscus tristiculus (Weise, 1929), Hyperaspis chevrolati Canepari, 1985, H. concolor Suffrian, 1843, H. hoffmannseggi (Gravenhorst, 1807), H. reppensis reppensis (Herbst, 1783), Coc- cidula rufa (Herbst, 1783), Coccinella (Coccinella) magnifi ca Redtenbacher, 1843, Tytthaspis phalerata (A. Costa, 1849), Oenopia lyncea agnata (Rosenhauser, 1847), Halyzia sedecimguttata (Linnaeus, 1758), Myrrha octodecimguttata octodecimguttata (Linnaeus, 1758), Calvia decemguttata (Linnaeus, 1758), Chilocorus similis (Rossi, 1790) and Exochomus nigromaculatus (Goeze, 1777)) are excluded from the Sardinian fauna. Key words: Coccinellidae, Sardinia, faunistics, new records, checklist. RIASSUNTO Contributo alla conoscenza dei Coccinellidae della Sardegna (Coleoptera) L'autore fornisce una lista aggiornata dei Coccinellidae di Sardegna; complessivamente sono segnalate con certezza 62 specie, mentre altre 19 specie (Scymnus (Mimopullus) fulvicollis (Mulsant, 1846), S. (M.) pharaonis Motschulsky, 1851, S. (Neopullus) haemorrhoidalis (Herbst, 1797), S. (N.) limbatus limbatus (Stephens, 1831), Nephus (Nephus) reunioni Fürsch, 1974, Scymniscus tristiculus (Weise, 1929), Hyperaspis chevrolati Canepari, 1985, H. -

Etude Des Communautés D'arthropodes Des Toitures
MASTER 2 Biodiversité, Ecologie, Evolution Parcours Gestion et Evolution de la Biodiversité Université de Lille, Sciences et Technologies Année 2018-2019 Etude des communautés d’arthropodes des toitures végétalisées d’Île-de-France Hamon Guillaume Encadrante : Lucile Dewulf Agence Régionale de la Biodiversité en Île-de-France 2 Remerciement Je tiens à remercier ici toutes les personnes qui ont contribué au succès de mon stage. Tout d’abord je tiens à remercier Julie Collombat-Dubois, directrice de l’Agence Régionale de la Biodiversité, pour m’avoir accepté en tant que stagiaire. A ma tutrice de stage Lucile Dewulf, pour ses conseils, pour la relecture de mon rapport et pour l’aide qu’elle m’a apporté. Je remercie également Maxime Zucca pour tout ce qu’il m’a appris, de m’avoir fait confiance et d’avoir pu t’accompagner faire du bagage. Un grand merci à toi Audrey Muratet pour toute l’aide que tu m’as apporté durant ce stage, pour avoir pris du temps pour m’aider dans mes analyses statistiques. Je remercie Colin Fontaine et Emmanuelle Porcher du MNHN d’avoir pris du temps pour nous aider dans le choix de nos analyses statistiques. Je tiens également à remercier toute l’équipe de l’ARB pour leur accueil, leur bonne humeur, et tout ce que j’ai pu apprendre à leurs côtés. Je tiens à remercier mes collègues stagiaires, Amandine Gallois et Christel Scagliola, ainsi que Morgane Bernard-Gat et Emir Kort, pour leur bonne humeur, leur aide et tous les bons moments que nous avons passé ensemble. -

Premières Rencontres Nationales Des Coccinellistes »
Actes des « Premières Rencontres Nationales des Coccinellistes » Coordinateurs : Jean-Pierre Coutanceau & Olivier Durand Institut de Biologie et d’Ecologie Appliquée de l’Université Catholique de l’Ouest Angers, 30 et 31 octobre 2014 HARMONIA COCCINELLES DU MONDE N°15 – DECEMBRE 2015 ISSN 2102-6769 Actes des « Premières rencontres nationales des Coccinellistes » - Angers, 2014 Liste des participants (par numérotation) : Jean-Pierre Coutanceau (1), Bernard Bal (2), Alexandra Magro (3), Thomas Hermant (4), Florence Brunet (5), Jeanine-Elisa Médélice (6), David Sauterey (7), Claire Coubard (8), Bruno Derolez (9), Bérénice Fassotte (10), Séverin Jouveau (11), Christopher Sénéchal (12), Santos Eizaguirre (13), Sylvain Barbier (14), Sophie Declercq (15), Olivier Durand (16), Gilbert Terrasse (17), Frédéric Noël (18), Pierre-Olivier Cochard (19), Romain Nattier (20), Henri Jurion (21). Manquent Vincent Nicolas et Johanna Villenave-Chasset (photo et silhouettes : O. Durand) HARMONIA - Coccinelles du monde, 15 (2015) Actes des « Premières rencontres nationales des Coccinellistes » - Angers, 2014 Hall d’accueil de l’IBEA de l’UCO (photos : O. Durand) HARMONIA - Coccinelles du monde, 15 (2015) Actes des « Premières rencontres nationales des Coccinellistes » - Angers, 2014 Allocutions de bienvenue de : M. Patrick Gillet, ancien directeur de l’IBEA et ex Vice-Recteur de l’UCO (à droite) et de M. Olivier Gabory, Directeur du CPIE Loire-Anjou Participants en échange et à l’écoute des exposés Bérénice Fassotte Vincent Nicolas (photos : O. Durand) HARMONIA -
Introduction to Identifying and Recording Ladybirds
Introduction to identifying and recording ladybirds Questions and Answers from Webinar on 7th May 2020 Thank you so much to everyone who participated in the webinar “Introduction to identifying and recording ladybirds”. We have enjoyed responding to all the questions that were posted. Please do get in contact if you have further comments or questions: [email protected] @UKLadybirds General ladybird ID questions Are the spots on the pronotum counted in the spot count? Or is it just the spots on the elytra? - Just the spots on the elytra (wing cases). What is the best way to identify harlequin ladybirds from others? -Harlequin ladybirds are generally bigger than most ladybirds but the combination of brown legs and the black “M” or solid black trapezoid shape on the pronotum are excellent ID features. Perhaps one of the most surprising features of harlequin ladybirds, and one that often causes confusion in identification, is the variation in colour of the wing cases. Most harlequin ladybirds are orange-red with black spots but some are black with red spots. How many species of inconspicuous ladybird are there in the UK? - We think that 22 inconspicuous ladybirds are now resident in the UK. What is the ladybird with the most spots in the world? - The one with the most spots that we know of is the potato ladybird, Epilachna vigintioctopunctata, which has 28 spots. This is not resident in the UK but has been reported here once. In the UK the 24- spot ladybird is the spottiest. Does the size change over time or between locations or the size is always have the same? - Adult ladybirds do not change in size or colour pattern.