A New Immunodiagnostic Test for the Detection of Southern Hemisphere Fish Residues in Processed Foods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Influences of Squid Ink Added to Low Salt Fermented Squid on Its Changes in Lactic Acid Bacteria
Korean J. Food & Nutr. Vol. 26. No. 4, 678~684 (2013) THE KOREAN JOURNAL OF http://dx.doi.org/10.9799/ksfan.2013.26.4.678 한국식품영양학회지 FOOD AND NUTRITION Influences of Squid Ink Added to Low Salt Fermented Squid on Its Changes in Lactic Acid Bacteria †Sung-Cheon Oh Dept. of Food & Pharmacy, Daewon University College, Jecheon 390-702, Korea 저염 오징어 젓갈의 숙성 중 오징어 먹즙 첨가가 젖산균의 변화에 미치는 영향 †오 성 천 대원대학교 제약식품계열 국문요약 오징어 먹즙을 첨가하지 않은 저염 오징어 젓갈은 숙성 발효에 따라 숙성 초기에 젖산균 총수가 급속히 증가하고, Leuconostoc이 증가하여 숙성 적기에 최대량을 나타내고 이후 서서히 감소하였다. Lactobacillus는 숙성 적기 이후의 총 균수의 대부분을 차지할 만큼 균수가 많아지므로 숙성 적기 이후 품질에 관여하는 것으로 보인다. Streptococcus와 Pediococcus는 숙성 적기까지 완만하게 증가하다가 이후 급격히 감소하였다. Yeast는 숙성 중기 이후에 검출되고, 숙성 말기에 급격히 증가하므로 부패에 주로 관여하는 것으로 보인다. 오징어 먹즙을 첨가한 저염 오징어 젓갈의 숙성발효에 따른 젖산균의 변화를 보면 총 균수는 숙성 중반까지 증가하다가 중반 이후 약간 감소하였으나, 최대치에 도달하는 기간은 무 첨가군에 비하여 길었고, 젖산균 중 Leuconostoc, Streptococcus 및 Pediococcus는 숙성 후반까지 증가하였다가 후반에 감소하는 반면에, Lactobacillus는 숙성 후반까지 계속 증가하였다. Yeast는 숙성 초기에는 거의 증가하지 않다가 숙성 중반 이후 증가하였는데, 이러한 경향은 무 첨가군과 유사하였으나 젖산균의 수가 무 첨가군에 비하여 적었다. 오징어 먹즙을 첨가한 저염 오징어 젓갈에서 모든 균들의 수가 감소되어 오징어 먹즙이 균들의 성장 을 억제하는 것으로 확인되었다. Key words: lactic acid bacteria, low salt fermented squid, squid ink, yeast, ripening Introduction is a dominant strain in the ripening stage regardless of the salt concentration although higher salt concentration reduces the Fermented squid is a fermented seafood product which contains bacterial count in an extract (Mori et al. -

Thermal Behavior, Microstructure and Protein Quality of Squid Fillets Dried
Food Control 36 (2014) 102e110 Contents lists available at ScienceDirect Food Control journal homepage: www.elsevier.com/locate/foodcont Thermal behavior, microstructure and protein quality of squid fillets dried by far-infrared assisted heat pump drying Yun Deng a, Yuegang Wang a, Jin Yue a,*, Zhenmin Liu b, Yuanrong Zheng b, Bingjun Qian b, Yu Zhong a, Yanyun Zhao a,c,* a SJTU-Bor Luh Food Safety Center, Department of Food Science and Technology, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, PR China b Dairy Research Institute, Bright Dairy & Food Co., Ltd., Bldg 2, No.1518, West Jiangchang Road, Shanghai 200436, PR China c Department of Food Science and Technology, 100 Wiegand Hall, Oregon State University, Corvallis, OR, USA article info abstract Article history: The effects of far-infrared assisted heat pump drying on the thermal behavior, microstructure and protein Received 6 May 2013 quality of squid fillets were studied. Heat pump drying (HPD) alone or in combination with far-infrared À Received in revised form radiation (FIR) at the power of 100, 500 and 800 W, 40 C and 2 m s 1 air velocity were compared. 3 August 2013 Nitrogen fractions, proteases activities, amino acid profile, microstructure, SDS-PAGE and protein quality Accepted 5 August 2013 of fresh and dried squid samples were examined. Results showed that glutamic acid, aspartic acid, lysine and isoleucine are the dominant amino acids in all samples. The total contents of essential amino acid Keywords: and non-essential amino acid, essential amino acid index, estimated protein efficiency ratio and bio- Heat pump drying fi > Far-infrared radiation logical value were not signi cantly different among all squid samples (P 0.05). -

A Practical Handbook for Determining the Ages of Gulf of Mexico And
A Practical Handbook for Determining the Ages of Gulf of Mexico and Atlantic Coast Fishes THIRD EDITION GSMFC No. 300 NOVEMBER 2020 i Gulf States Marine Fisheries Commission Commissioners and Proxies ALABAMA Senator R.L. “Bret” Allain, II Chris Blankenship, Commissioner State Senator District 21 Alabama Department of Conservation Franklin, Louisiana and Natural Resources John Roussel Montgomery, Alabama Zachary, Louisiana Representative Chris Pringle Mobile, Alabama MISSISSIPPI Chris Nelson Joe Spraggins, Executive Director Bon Secour Fisheries, Inc. Mississippi Department of Marine Bon Secour, Alabama Resources Biloxi, Mississippi FLORIDA Read Hendon Eric Sutton, Executive Director USM/Gulf Coast Research Laboratory Florida Fish and Wildlife Ocean Springs, Mississippi Conservation Commission Tallahassee, Florida TEXAS Representative Jay Trumbull Carter Smith, Executive Director Tallahassee, Florida Texas Parks and Wildlife Department Austin, Texas LOUISIANA Doug Boyd Jack Montoucet, Secretary Boerne, Texas Louisiana Department of Wildlife and Fisheries Baton Rouge, Louisiana GSMFC Staff ASMFC Staff Mr. David M. Donaldson Mr. Bob Beal Executive Director Executive Director Mr. Steven J. VanderKooy Mr. Jeffrey Kipp IJF Program Coordinator Stock Assessment Scientist Ms. Debora McIntyre Dr. Kristen Anstead IJF Staff Assistant Fisheries Scientist ii A Practical Handbook for Determining the Ages of Gulf of Mexico and Atlantic Coast Fishes Third Edition Edited by Steve VanderKooy Jessica Carroll Scott Elzey Jessica Gilmore Jeffrey Kipp Gulf States Marine Fisheries Commission 2404 Government St Ocean Springs, MS 39564 and Atlantic States Marine Fisheries Commission 1050 N. Highland Street Suite 200 A-N Arlington, VA 22201 Publication Number 300 November 2020 A publication of the Gulf States Marine Fisheries Commission pursuant to National Oceanic and Atmospheric Administration Award Number NA15NMF4070076 and NA15NMF4720399. -

Jolanta KEMPTER*, Maciej KIEŁPIŃSKI, Remigiusz PANICZ, and Sławomir KESZKA
ACTA ICHTHYOLOGICA ET PISCATORIA (2016) 46 (4): 287–291 DOI: 10.3750/AIP2016.46.4.02 MICROSATELLITE DNA-BASED GENETIC TRACEABILITY OF TWO POPULATIONS OF SPLENDID ALFONSINO, BERYX SPLENDENS (ACTINOPTERYGII: BERYCIFORMES: BERYCIDAE)—PROJECT CELFISH—PART 2 Jolanta KEMPTER*, Maciej KIEŁPIŃSKI, Remigiusz PANICZ, and Sławomir KESZKA Division of Aquaculture, West Pomeranian University of Technology, Szczecin, Kazimierza Krolewicza 4, 71-550 Szczecin, Poland Kempter J., Kiełpinski M., Panicz R., Keszka S. 2016. Microsatellite DNA-based genetic traceability of two populations of splendid alfonsino, Beryx splendens (Actinopterygii: Beryciformes: Berycidae)— Project CELFISH—Part 2. Acta Ichthyol. Piscat. 46 (4): 287–291. Background. The study is a contribution to Project CELFISH which involves genetic identifi cation of populations of fi sh species presenting a particular economic importance or having a potential to be used in the so-called commercial substitutions. The EU fi sh trade has been showing a distinct trend of more and more fi sh species previously unknown to consumers being placed on the market. Molecular assays have become the only way with which to verify the reliability of exporters. This paper is aimed at pinpointing genetic markers with which to label and differentiate between two populations of splendid alfonsino, Beryx splendens Lowe, 1834, a species highly attractive to consumers in Asia and Oceania due to the meat taste and low fat content. Material and methods. DNA was isolated from fragments of fi ns collected at local markets in Japan (MJ) (n = 10) and New Zealand (MNZ) (n = 18). The rhodopsin gene (RH1) fragment and 16 microsatellite DNA fragments (SSR) were analysed in all the individuals. -

Table S1.Xlsx
Bone type Bone type Taxonomy Order/series Family Valid binomial Outdated binomial Notes Reference(s) (skeletal bone) (scales) Actinopterygii Incertae sedis Incertae sedis Incertae sedis †Birgeria stensioei cellular this study †Birgeria groenlandica cellular Ørvig, 1978 †Eurynotus crenatus cellular Goodrich, 1907; Schultze, 2016 †Mimipiscis toombsi †Mimia toombsi cellular Richter & Smith, 1995 †Moythomasia sp. cellular cellular Sire et al., 2009; Schultze, 2016 †Cheirolepidiformes †Cheirolepididae †Cheirolepis canadensis cellular cellular Goodrich, 1907; Sire et al., 2009; Zylberberg et al., 2016; Meunier et al. 2018a; this study Cladistia Polypteriformes Polypteridae †Bawitius sp. cellular Meunier et al., 2016 †Dajetella sudamericana cellular cellular Gayet & Meunier, 1992 Erpetoichthys calabaricus Calamoichthys sp. cellular Moss, 1961a; this study †Pollia suarezi cellular cellular Meunier & Gayet, 1996 Polypterus bichir cellular cellular Kölliker, 1859; Stéphan, 1900; Goodrich, 1907; Ørvig, 1978 Polypterus delhezi cellular this study Polypterus ornatipinnis cellular Totland et al., 2011 Polypterus senegalus cellular Sire et al., 2009 Polypterus sp. cellular Moss, 1961a †Scanilepis sp. cellular Sire et al., 2009 †Scanilepis dubia cellular cellular Ørvig, 1978 †Saurichthyiformes †Saurichthyidae †Saurichthys sp. cellular Scheyer et al., 2014 Chondrostei †Chondrosteiformes †Chondrosteidae †Chondrosteus acipenseroides cellular this study Acipenseriformes Acipenseridae Acipenser baerii cellular Leprévost et al., 2017 Acipenser gueldenstaedtii -

© Iccat, 2007
A5 By-catch Species APPENDIX 5: BY-CATCH SPECIES A.5 By-catch species By-catch is the unintentional/incidental capture of non-target species during fishing operations. Different types of fisheries have different types and levels of by-catch, depending on the gear used, the time, area and depth fished, etc. Article IV of the Convention states: "the Commission shall be responsible for the study of the population of tuna and tuna-like fishes (the Scombriformes with the exception of Trichiuridae and Gempylidae and the genus Scomber) and such other species of fishes exploited in tuna fishing in the Convention area as are not under investigation by another international fishery organization". The following is a list of by-catch species recorded as being ever caught by any major tuna fishery in the Atlantic/Mediterranean. Note that the lists are qualitative and are not indicative of quantity or mortality. Thus, the presence of a species in the lists does not imply that it is caught in significant quantities, or that individuals that are caught necessarily die. Skates and rays Scientific names Common name Code LL GILL PS BB HARP TRAP OTHER Dasyatis centroura Roughtail stingray RDC X Dasyatis violacea Pelagic stingray PLS X X X X Manta birostris Manta ray RMB X X X Mobula hypostoma RMH X Mobula lucasana X Mobula mobular Devil ray RMM X X X X X Myliobatis aquila Common eagle ray MYL X X Pteuromylaeus bovinus Bull ray MPO X X Raja fullonica Shagreen ray RJF X Raja straeleni Spotted skate RFL X Rhinoptera spp Cownose ray X Torpedo nobiliana Torpedo -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Spanish Mackerel J
2.1.10.6 SSM CHAPTER 2.1.10.6 AUTHORS: LAST UPDATE: ATLANTIC SPANISH MACKEREL J. VALEIRAS and E. ABAD Sept. 2006 2.1.10.6 Description of Atlantic Spanish Mackerel (SSM) 1. Names 1.a Classification and taxonomy Species name: Scomberomorus maculatus (Mitchill 1815) ICCAT species code: SSM ICCAT names: Atlantic Spanish mackerel (English), Maquereau espagnol (French), Carita del Atlántico (Spanish) According to Collette and Nauen (1983), the Atlantic Spanish mackerel is classified as follows: • Phylum: Chordata • Subphylum: Vertebrata • Superclass: Gnathostomata • Class: Osteichthyes • Subclass: Actinopterygii • Order: Perciformes • Suborder: Scombroidei • Family: Scombridae 1.b Common names List of vernacular names used according to ICCAT, FAO and Fishbase (www.fishbase.org). The list is not exhaustive and some local names might not be included. Barbados: Spanish mackerel. Brazil: Sororoca. China: ᶷᩬ㤿㩪. Colombia: Sierra. Cuba: Sierra. Denmark: Plettet kongemakrel. Former USSR: Ispanskaya makrel, Korolevskaya pyatnistaya makrel, Pyatnistaya makrel. France: Thazard Atlantique, Thazard blanc. Germany: Gefleckte Königsmakrele. Guinea: Makréni. Italy: Sgombro macchiato. Martinique: Taza doré, Thazard tacheté du sud. Mexico: Carite, Pintada, Sierra, Sierra común. Poland: Makrela hiszpanska. Portugal: Serra-espanhola. Russian Federation: Ispanskaya makrel, Korolevskaya pyatnistaya makrel, Pyatnistaya makrel; ɦɚɤɪɟɥɶ ɢɫɩɚɧɫɤɚɹ. South Africa: Spaanse makriel, Spanish mackerel. Spain: Carita Atlántico. 241 ICCAT MANUAL, 1st Edition (January 2010) Sweden: Fläckig kungsmakrill. United Kingdom: Atlantic spanish mackerel. United States of America: Spanish mackerel. Venezuela: Carite, Sierra pintada. 2. Identification Figure 1. Drawing of an adult Atlantic Spanish mackerel (by A. López, ‘Tokio’). Characteristics of Scomberomorus maculatus (see Figure 1 and Figure 2) Atlantic Spanish mackerel is a small tuna species. Maximum size is 91 cm fork length and 5.8 kg weight (IGFA 2001). -

A Preliminary Global Assessment of the Status of Exploited Marine Fish and Invertebrate Populations
A PRELIMINARY GLOBAL ASSESSMENT OF THE STATUS OF EXPLOITED MARINE FISH AND INVERTEBRATE POPULATIONS June 30 2018 A PRELIMINARY GLOBAL ASSESSMENT OF THE STATUS OF EXPLOITED MARINE FISH AND INVERTEBRATE POPULATIONS Maria. L.D. Palomares, Rainer Froese, Brittany Derrick, Simon-Luc Nöel, Gordon Tsui Jessika Woroniak Daniel Pauly A report prepared by the Sea Around Us for OCEANA June 30, 2018 A PRELIMINARY GLOBAL ASSESSMENT OF THE STATUS OF EXPLOITED MARINE FISH AND INVERTEBRATE POPULATIONS Maria L.D. Palomares1, Rainer Froese2, Brittany Derrick1, Simon-Luc Nöel1, Gordon Tsui1, Jessika Woroniak1 and Daniel Pauly1 CITE AS: Palomares MLD, Froese R, Derrick B, Nöel S-L, Tsui G, Woroniak J, Pauly D (2018) A preliminary global assessment of the status of exploited marine fish and invertebrate populations. A report prepared by the Sea Around Us for OCEANA. The University of British Columbia, Vancouver, p. 64. 1 Sea Around Us, Institute for the Oceans and Fisheries, University of British Columbia, 2202 Main Mall, Vancouver BC V6T1Z4 Canada 2 Helmholtz Centre for Ocean Research GEOMAR, Düsternbrooker Weg 20, 24105 Kiel, Germany TABLE OF CONTENTS Executive Summary 1 Introduction 2 Material and Methods 3 − Reconstructed catches vs official catches 3 − Marine Ecoregions vs EEZs 3 − The CMSY method 5 Results and Discussion 7 − Stock summaries reports 9 − Problematic stocks and sources of bias 14 − Stocks in the countries where OCEANA operates 22 − Stock assessments on the Sea Around Us website 31 − The next steps 32 Acknowledgements 33 References 34 Appendices I. List of marine ecoregions by EEZ 37 II. Summaries of number of stock by region and 49 by continent III. -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -
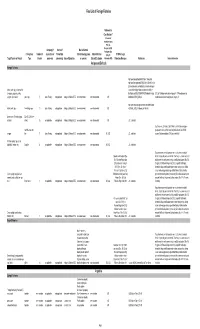
2018 Final LOFF W/ Ref and Detailed Info
Final List of Foreign Fisheries Rationale for Classification ** (Presence of mortality or injury (P/A), Co- Occurrence (C/O), Company (if Source of Marine Mammal Analogous Gear Fishery/Gear Number of aquaculture or Product (for Interactions (by group Marine Mammal (A/G), No RFMO or Legal Target Species or Product Type Vessels processor) processing) Area of Operation or species) Bycatch Estimates Information (N/I)) Protection Measures References Detailed Information Antigua and Barbuda Exempt Fisheries http://www.fao.org/fi/oldsite/FCP/en/ATG/body.htm http://www.fao.org/docrep/006/y5402e/y5402e06.htm,ht tp://www.tradeboss.com/default.cgi/action/viewcompan lobster, rock, spiny, demersal fish ies/searchterm/spiny+lobster/searchtermcondition/1/ , (snappers, groupers, grunts, ftp://ftp.fao.org/fi/DOCUMENT/IPOAS/national/Antigua U.S. LoF Caribbean spiny lobster trap/ pot >197 None documented, surgeonfish), flounder pots, traps 74 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G AndBarbuda/NPOA_IUU.pdf Caribbean mixed species trap/pot are category III http://www.nmfs.noaa.gov/pr/interactions/fisheries/tabl lobster, rock, spiny free diving, loops 19 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G e2/Atlantic_GOM_Caribbean_shellfish.html Queen conch (Strombus gigas), Dive (SCUBA & free molluscs diving) 25 not applicable not applicable Antigua & Barbuda EEZ none documented none documented A/G U.S. trade data Southeastern U.S. Atlantic, Gulf of Mexico, and Caribbean snapper- handline, hook and grouper and other reef fish bottom longline/hook-and-line/ >5,000 snapper line 71 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented N/I, A/G U.S. -

Targeted Review of Biological and Ecological Information from Fisheries Research in the South East Marine Region
TARGETED REVIEW OF BIOLOGICAL AND ECOLOGICAL INFORMATION FROM FISHERIES RESEARCH IN THE SOUTH EAST MARINE REGION FINAL REPORT B. D. Bruce, R. Bradford, R. Daley, M. Green and K. Phillips December 2002 Client: National Oceans Office Targeted review of biological and ecological information from fisheries research in the South East Marine Region Final Report B. D. Bruce, R. Bradford, R. Daley M. Green and K. Phillips* CSIRO Marine Research, Hobart * National Oceans Office December 2002 2 Table of Contents: Table of Contents:...................................................................................................................................3 Introduction.............................................................................................................................................5 Objective of review.............................................................................................................................5 Structure of review..............................................................................................................................5 Format.................................................................................................................................................6 General ecological/biological issues and uncertainties for the South East Marine Region ....................9 Specific fishery and key species accounts ............................................................................................10 South East Fishery (SEF) including the South East Trawl