M Richämdl. Longini. 2,200
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety Data Sheet 1
Safety Data Sheet 1. Waters Corporation EU: Waters Chromatography Europe B.V. FOR CHEMICAL EMERGENCY 34 Maple Street Mon Plaisir 12, Postbus 215 24 Hours per Day, 7 Days per Week Milford, MA 01757 U.S.A. 4870 AE Etten-Leur N B, The Netherlands Call CHEMTREC 1-800-424-9300 Tel: +1 508 478-2000 Tel: +31 76 508 1800 Outside USA & Canada (collect calls accepted) FAX: +1 508 872-1990 Waters Technologies Ireland Ltd +1 703-741-5970 www.waters.com Drinagh, Wexford, Ireland MSDS email inquiries: Tel: +353 53 91 60400 [email protected] 1. IDENTIFICATION Product: MassPREP™ Calibration Mix - DIOS Low Mass, PN 186002820 MSDS #: 715001038 Product Use: For laboratory use only. Date: Rev B, December 1, 2014 2. HAZARDS IDENTIFICATION: The calibration kit mixtures are not classified as hazardous per GHS and OSHA. Not dangerous according to the criteria set by the European Union (EU); not listed in Table 3.1 Annex VI of regulation 1272/2008/EU, as amended. The mixtures are not hazardous in the form in which they are placed on the market and under the normal and recommended conditions of storage and use. The small quantities supplied in our products are unlikely to cause severe or immediate health effects. However idodide compounds can be irritating to eyes and skin. Use only as directed and in accordance with safe laboratory practices. 3. COMPOSITION/INFORMATION ON INGREDIENTS: EXPOSURE LIMITS CAS EC % by OSHA ACGIH EU CHEMICAL INGREDIENT NAME NUMBER NUMBER Weight IOELV Polyethylene Glycol (PEG) 25322-68-3 203-989-9 100 NA NE NA Sodium Iodide 7681-82-5 231-679-3 99.9 NA 0.01 ppm NA (iodides) Cesium Iodide 7789-17-5 232-145-2 99.9 NA 0.01 ppm NA (iodides) Rubidium Iodide 7790-29-6 232-198-1 99.9 NA 0.01 ppm NA (iodides) Notes: Kit contains dried calibration mixtures: Polyethylene Glycol in a range of molecular weights (MW 200, 600, 1000); 50 ug of Sodium Iodide; 50 ug Rubidium Iodide; and 12.5 ug Cesium Iodide. -

2 & 4. %Z-E Cézé,26
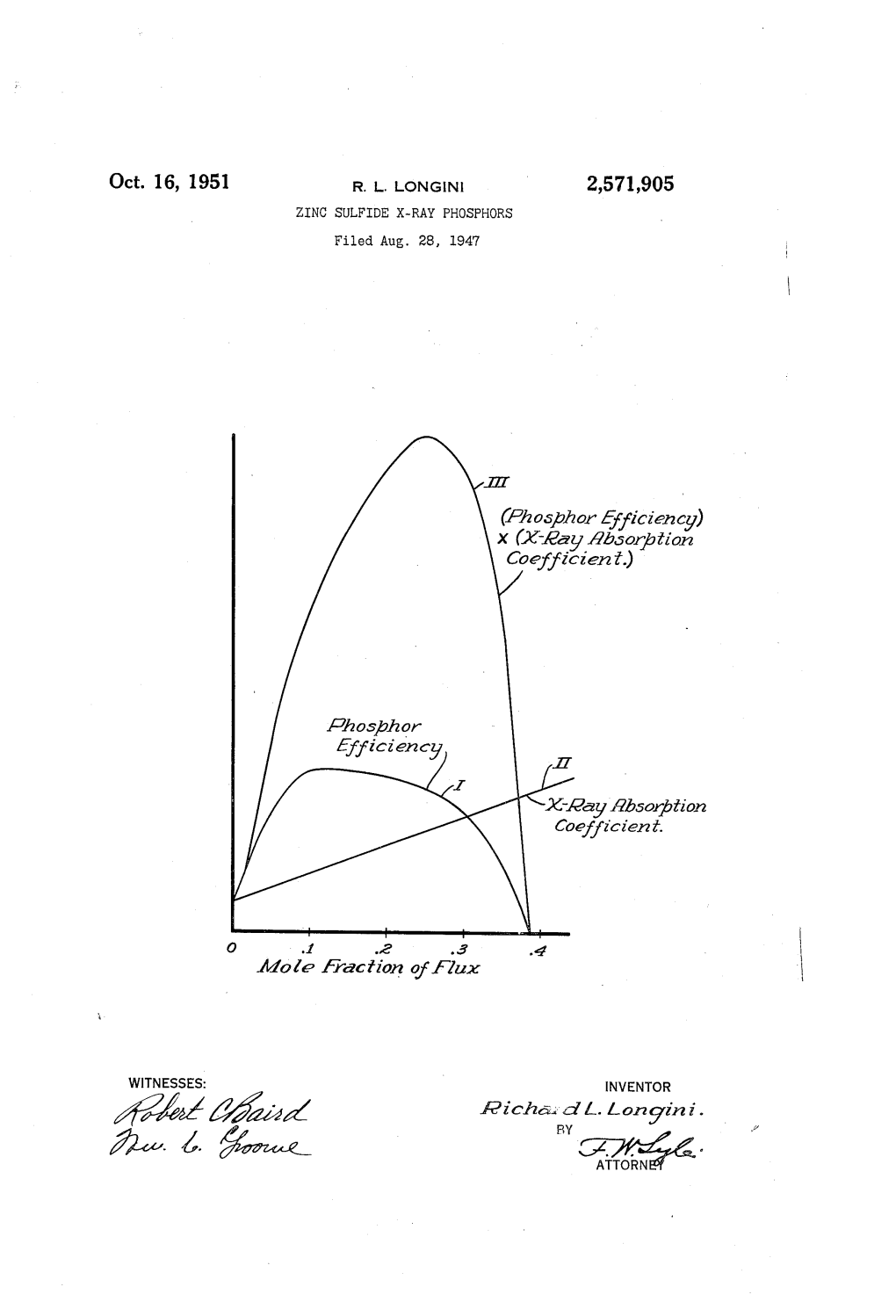
Oct. 16, 1951 R. L. LONGIN 2,571,905 ZINC SULFIDE X-RAY PHOSFHORS Filed Aug. 28, 1947 777 (AZhosphor Afficiency) X (Kay Asorptiora Coefficieri.) Ahosphor Afficiency 2C-Alay Absorption Coefficier? . O ..f ..a 3 Azo de Araction of A Zux WITNESSES: INVENTOR 42% 42-az Aicha. d.?. longini. 2 & 4. %z-e RY Cézé,26. Patented Oct. 16, 1951 -3. 2,571,905 UNITED STATES PATENT office 2,571,905 ZINC SULFIDEX-RAY PHosPHORs Richard L. Longini, Pittsburgh, Pa., assignor to Westinghouse Electric Corporation, East Pitts burgh, Pa., a corporation of Pennsylvania, Application August 28, 1947, Serial No. 71,113 4 Claims. (C. 252-301.6) 1. 2 My invention relates to materials which be In making measurements of the luminous effi come fluorescent to produce visible light under ciency of Such materials, I have found that while the impact of X-rays and, in particular, relates Zinc sulphide produces a much larger yield of to a method of combining different substances visible radiation than does calcium tungstate for to produce a maximum yield of visible radia a given absorption of X-ray energy, the luminous tion for a given X-ray energization. intensity of the zinc sulphide screens is made In the medical and other X-ray arts, it is fre undesirably low because zinc sulphide has a very quently desirable to make visible the X-ray pat low absorption coefficient. I have, however, found terns produced by irradiating various objects that this difficulty can be corrected by admixing Which are opaque to visible radiation by streams 10 With the zinc sulphide an ancillary material or of X-rays, and screens covered by fluorescent ma “flux' in the form of an alkali halide. -

The Structure and Stability of Simple Tri-Iodides
THE STRUCTURE AND STABILITY OF SIMPLE TRI -IODIDES by ANTHONY JOHN THOMPSON FINNEY B.Sc.(Hons.) submitted in fulfilment of the requirements for the Degree of Doctor of Philosophy UNIVERSITY OF TASMANIA HOBART OCTOBER, 1973 . r " • f (i) Frontispiece (reproduced as Plate 6 - 1, Chapter 1) - two views of a large single crystal of KI 3 .H20. The dimensions of this specimen were approximately 3.0 cm x 1.5 cm x 0.5 cm. • - - . ;or • - This thesis contains no material which has been accepted for the award of any other degree or diploma in any University, and to the best of my knowledge and belief, this thesis contains no copy or paraphrase of material previously published or written by another person, except where reference is made in the text of this thesis. Anthony John Finney Contents page Abstract (iv) Acknowledgements (vii) Chapter 1 - The Structure and Stability of Simple 1 Tri-iodides Chapter 2 - The Theoretical Basis of X-Ray Structural 32 Analysis Chapter 3 - The Crystallographic Program Suite 50 Chapter 4 - The Refinement of the Structure of NH I 94 4 3 Chapter 5 - The Solution of the Structure of RbI 115 3 Chapter 6 - The Solution of the Structure of KI 3 .1120 135 Chapter 7 Discussion of the Inter-relation of 201 Structure and Stability Bibliography 255 Appendix A - Programming Details 267 Appendix B - Density Determinations 286 Appendix C - Derivation of the Unit Cell Constants of 292 KI .H 0 3 2 Appendix D - I -3 force constant Calculation 299 Appendix E - Publications 311 ( iv) THE STRUCTURE AND STABILITY OF SIMPLE TRI-IODIDES Abstract In this work the simple tri-iodides are regarded as being those in which the crystal lattice contains only cations, tri-iodide anions and possibly solvate molecules. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Durham E-Theses
Durham E-Theses Halogen and interhalogen adducts of substituted amido-ions Britton, G.C. How to cite: Britton, G.C. (1979) Halogen and interhalogen adducts of substituted amido-ions, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/8946/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk HALOGEN AND INTERHALOGEN ABDUCTS OF SUBSTITUTED AMI DO-IONS A THESIS PRESENTED BY G. C. BRITTON FOR THE DEGREE OF MASTER OF SCIENCE" OF THE UNIVERSITY OF DURHAM DEPARTMENT OF CHEMISTRY UNIVERSITY OF DURHAM The copyright of this thesis rests with the author. JUNE 1979 No quotation from it should be published without Unive. his prior written consent and information derived !*• SCIENCE "> 2 9 NOV V) from it should be acknowledged. SECTION LibraH ABSTRACT The reaction between N-dimethylchloramine and iodomethane - + which yields the species (CHj)4N (CH^)2N(lCl)2~ - has been investigated with a view to elucidating possible mechanisms, and a number of analagous and related compounds have been prepared. -

Download Article (PDF)
An X-Ray Diffraction Study on the Structure of Concentrated Aqueous Caesium Iodide and Lithium Iodide Solutions Yusuke Tamura, Toshio Yamaguchi *, Isao Okada. and Hitoshi Ohtaki Department of Electronic Chemistry, Tokyo Institute of Technology, Nagatsuta, Midori-ku, Yokohama 227, Japan Z. Naturforsch. 42 a, 367-376 (1987); received December 8, 1986 X-Ray scattering measurements of 2.78 and 5.56 molal aqueous solutions of caesium iodide and 2.78 and 6.05 molal lithium iodide were carried out at 293 and 343 K Differences in the radial distribution functions (DRDFs) have been obtained between the caesium iodide and lithium iodide solutions of similar composition, the latter being taken as a reference for the data analysis of the former. The DRDFs show a peak arising from Cs-I contact-ion-pairs at 390 pm for all the caesium iodide solutions. The hydration structure of the caesium and iodide ions has been revealed. Effects of the concentration and temperature on the formation of ion-pairs and on the hydration structure of the ions are discussed. 1. Introduction cept Li+ increases. However, the above studies have given only indirect information with respect to The structure of electrolyte solutions, in partic- structural properties of hydrated ions. Recently, ular alkali halide solutions, has widely been in- Heinzinger et al. have obtained direct structural vestigated by means of X-ray and neutron diffrac- information on the effect of temperature and pres- tion [1] and Monte Carlo and molecular dynamics sure on the hydration shells of Li+ and I- and on the simulations [2], from which the structure and prop- bulk water by means of molecular dynamics (MD) erties of hydrated ions have been revealed. -

Combined Tof-SIMS and XPS Characterization of 304L Surface After Interaction with Caesium Iodide Under PWR Severe Accident Conditions D
Combined ToF-SIMS and XPS characterization of 304L surface after interaction with caesium iodide under PWR severe accident conditions D. Obada, Anne-Sophie Mamede, Nicolas Nuns, Anne-Cécile Grégoire, Laurent Gasnot To cite this version: D. Obada, Anne-Sophie Mamede, Nicolas Nuns, Anne-Cécile Grégoire, Laurent Gasnot. Com- bined ToF-SIMS and XPS characterization of 304L surface after interaction with caesium iodide under PWR severe accident conditions. Applied Surface Science, Elsevier, 2018, 459, pp.23-31. 10.1016/j.apsusc.2018.07.212. hal-02335537 HAL Id: hal-02335537 https://hal.univ-lille.fr/hal-02335537 Submitted on 17 Jul 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Combined ToF-SIMS and XPS characterization of 304L surface after interaction with caesium iodide under PWR severe accident conditions Dorel Obada1, Anne-Sophie Mamede2, Nicolas Nuns3, Anne-Cécile Grégoire1, Laurent Gasnot4 1 Institut de Radioprotection et de Sûreté Nucléaire, Pôle Sûreté Nucléaire, CEN Cadarache, Saint Paul lez Durance, F-13115, France ; [email protected] ; anne- [email protected] 2 Univ. Lille, CNRS, ENSCL, Centrale Lille, Univ. Artois, UMR 8181 – UCCS – Unité de Catalyse et Chimie du Solide, F-59000 Lille, France ; [email protected] 3 Univ. -

Case No COMP/M.7393 - ALBEMARLE/ ROCKWOOD
EN Case No COMP/M.7393 - ALBEMARLE/ ROCKWOOD Only the English text is available and authentic. REGULATION (EC) No 139/2004 MERGER PROCEDURE Article 6(1)(b) NON-OPPOSITION Date: 13/11/2014 In electronic form on the EUR-Lex website under document number 32014M7393 Office for Publications of the European Union L-2985 Luxembourg EUROPEAN COMMISSION Brussels, 13.11.2014 C(2014) 8615 final In the published version of this decision, some information has been omitted pursuant to Article 17(2) of Council Regulation (EC) No 139/2004 concerning non-disclosure of business secrets and other confidential information. The omissions are shown thus […]. Where possible the information PUBLIC VERSION omitted has been replaced by ranges of figures or a general description. MERGER PROCEDURE To the notifying party: Dear Sir/Madam, Subject: Case M.7393 – Albemarle/ Rockwood Commission decision pursuant to Article 6(1)(b) of Council Regulation No 139/20041 and article 57 of the EEA agreement (1) On 9 October 2014, the European Commission received notification of a proposed concentration pursuant to Article 4 of the Merger Regulation by which Albemarle Corporation (Albemarle, US) acquires within the meaning of Article 3(1)(b) of the Merger Regulation control of the whole of Rockwood Holdings, Inc. (Rockwood, US) by way of purchase of securities2, hereinafter referred to as "the proposed transaction" or the "Transaction". Albemarle and Rockwood are designated hereinafter as the "Parties". 1. THE PARTIES (2) Albemarle is a global company which develops, manufactures and markets specialty chemicals across a diverse range of end markets including the petroleum refining, consumer electronics, plastics/packaging, construction, automotive, lubricants, pharmaceuticals, crop protection, food safety and custom chemistry services markets. -

Formamidinium Lead Halide Perovskites Zhiping Wang, Qianqian Lin, Francis P
ARTICLES PUBLISHED: 14 AUGUST 2017 | VOLUME: 2 | ARTICLE NUMBER: 17135 Ecient ambient-air-stable solar cells with 2D–3D heterostructured butylammonium-caesium- formamidinium lead halide perovskites Zhiping Wang, Qianqian Lin, Francis P. Chmiel, Nobuya Sakai, Laura M. Herz and Henry J. Snaith* Perovskite solar cells are remarkably ecient; however, they are prone to degradation in water, oxygen and ultraviolet light. Cation engineering in 3D perovskite absorbers has led to reduced degradation. Alternatively, 2D Ruddlesden–Popper layered perovskites exhibit improved stability, but have not delivered ecient solar cells so far. Here, we introduce n-butylammonium cations into a mixed-cation lead mixed-halide FA0.83Cs0.17Pb(IyBr1 y)3 3D perovskite. We observe the formation of 2D perovskite platelets, interspersed between highly orientated 3D perovskite− grains, which suppress non-radiative charge recombination. We investigate the relationship between thin-film composition, crystal alignment and device performance. Solar cells with an optimal butylammonium content exhibit average stabilized power conversion eciency of 17.5 1.3% with a 1.61-eV-bandgap perovskite and 15.8 0.8% with a 1.72-eV-bandgap perovskite. The stability under simulated± sunlight is also enhanced. Cells sustain 80% of their± ‘post burn-in’ eciency after 1,000 h in air, and close to 4,000 h when encapsulated. rganic–inorganic metal halide perovskites have emerged as employing layered perovskite compounds have exhibited relatively one of the most promising next-generation photovoltaic low efficiency (PCE < 10%) (refs 20,22), assumed to be due to the materials due to their outstanding properties, such as inhibition of out-of-plane charge transport by the insulating spacer O 1,2 3 tunable bandgap , long electron–hole diffusion lengths , low cations. -

Controlling and Exploiting the Caesium Effect in Palladium Catalysed Coupling Reactions
Controlling and exploiting the caesium effect in palladium catalysed coupling reactions Thomas J. Dent Submitted in accordance with the requirements for the degree of Doctor of Philosophy The University of Leeds School of Chemistry May 2019 i The candidate confirms that the work submitted is his own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the report may be published without proper acknowledgement The right of Thomas Dent to be identified as Author of this work has been asserted by him in accordance with the Copyright, Designs and Patents Act 1988. © 2019 The University of Leeds and Thomas J. Dent ii Acknowledgements This project could not have been completed without the help of several individuals who’ve helped guide the project into the finished article. First and foremost I’d like to thank Dr. Bao Nguyen his support, useful discussions and the ability to sift through hundreds of experiments of kinetic data to put together a coherent figure. My writing has come a long way from my transfer report, so all the comments and suggestions seem to have mostly not been in vain. To Paddy, the discussions relating to the NMR studies and anything vaguely inorganic were incredibly useful, and provided me with data that supported our hypothesis with more direct evidence than just the reaction monitoring experiments. Rob, I really enjoyed my time at AZ and your support during my time there was incredibly useful so I could maximise my short secondment when I was getting more results than I knew what to do with. -

Provisional Peer-Reviewed Toxicity Values for Rubidium Compounds
EPA/690/R-16/012F l Final 9-02-2016 Provisional Peer-Reviewed Toxicity Values for Rubidium Compounds (CASRN 7440-17-7, Rubidium) (CASRN 7791-11-9, Rubidium Chloride) (CASRN 1310-82-3, Rubidium Hydroxide) (CASRN 7790-29-6, Rubidium Iodide) Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 AUTHORS, CONTRIBUTORS, AND REVIEWERS CHEMICAL MANAGERS Puttappa R. Dodmane, BVSc&AH, MVSc, PhD National Center for Environmental Assessment, Cincinnati, OH Scott C. Wesselkamper, PhD National Center for Environmental Assessment, Cincinnati, OH DRAFT DOCUMENT PREPARED BY SRC, Inc. 7502 Round Pond Road North Syracuse, NY 13212 PRIMARY INTERNAL REVIEWER Paul G. Reinhart, PhD, DABT National Center for Environmental Assessment, Research Triangle Park, NC This document was externally peer reviewed under contract to: Eastern Research Group, Inc. 110 Hartwell Avenue Lexington, MA 02421-3136 Questions regarding the contents of this PPRTV assessment should be directed to the EPA Office of Research and Development’s National Center for Environmental Assessment, Superfund Health Risk Technical Support Center (513-569-7300). ii Rubidium Compounds TABLE OF CONTENTS COMMONLY USED ABBREVIATIONS AND ACRONYMS .................................................. iv BACKGROUND .............................................................................................................................1 DISCLAIMERS ...............................................................................................................................1 -

“M/S. AJANTA PHARMA LTD.”
APPLICATION FOR ENVIRONMENTAL CLEARANCE OF PROPOSED EXPANSION OF ACTIVE PHARMACEUTICAL INGREDIENTS (API), MANUFACTURING UNIT “M/s. AJANTA PHARMA LTD.” 11KM Stone, Gut No. 378, Plot No 8, Aurangabad –Pune Highway, Village-Waluj, Taluka. Gangapur, District. Aurangabad- 431133. FORM 1 Submitted to Expert Appraisal Committee (Industry-2), MoEFCC, New Delhi Submitted by M/s. AJANTA PHARMA LTD. Environmental Consultant: Building Environment (India) Pvt. Ltd Dakshina Building, Office No. 401, Plot No. 2, Sector-11, CBD Belapur, Navi Mumbai- 400 614 January, 2018. Form 1 for proposed Expansion of API Manufacturing Industry “M/s. Ajanta Pharma Ltd.” at Waluj Village, Taluka-Gangapur, District- Aurangabad, Maharashtra. Form – 1 (I) Basic Information:- Sr. Items Details No. 1. Name of the project Proposed expansion of Active Pharmaceutical Ingredients (API) Manufacturing Industry “M/s. Ajanta Pharma Ltd.” 2. S. No. in the schedule Category 5f as per EIA Notification 2006 & amendments 3. Proposed capacity/ area/ length/ Industry is already engaged in manufacturing 85 nos. of tonnage to be handled/ command API and having production capacity 21.042 MT/Month. area/lease area/ number of wells to In the proposed expansion industry will manufacture 256 be drilled nos. API including existing API. The total production capacity after expansion would remain same as existing i.e. 21.042 MT/Month. Annexure-1 : Details of existing & proposed API 4. New / Expansion / Modernization Expansion. Annexure -2: EC letter of existing unit 5. Existing Capacity/ Area etc. Industry is already engaged in manufacturing 85 nos. of API and having production 21.042 MT/Month. Annexure-3 : Details of existing API 6.