Intravascular Ultrasound-Guided Internal Carotid Artery Stenting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endarterectomy Versus Stenting for Stroke Prevention
Open Access Review Stroke Vasc Neurol: first published as 10.1136/svn-2018-000146 on 24 February 2018. Downloaded from Endarterectomy versus stenting for stroke prevention A Ross Naylor To cite: Naylor AR. ABSTRACT stenosis,1 2 using the NASCET method for Endarterectomy versus stenting The European Society for Vascular Surgery (ESVS) has measuring carotid stenosis severity.2 Subgroup for stroke prevention. Stroke recently prepared updated guidelines for the management analyses suggested that it was possible to iden- and Vascular Neurology 2018;0: of patients with symptomatic and asymptomatic e000146. doi:10.1136/svn- tify certain imaging/clinical features that atherosclerotic carotid artery disease, with specific 2018-000146 were associated with a higher risk of stroke on reference to the roles of best medical therapy, carotid BMT.3 Clinical features of increased benefit endarterectomy (CEA) and carotid artery stenting (CAS). In conferred by CEA include: increasing age Received 15 January 2018 symptomatic patients, there is a drive towards performing Accepted 4 February 2018 carotid interventions as soon as possible after onset of (especially patients aged >75 years), recency symptoms. This is because it is now recognised that the of symptoms, male sex, hemispheric versus highest risk period for recurrent stroke is the first 7–14 ocular symptoms, cortical versus lacunar 3 days after onset of symptoms. The guidelines advise that stroke and increasing medical comorbidities. there is a role for both CEA and CAS, but the levels of Imaging -

Having an Aortic Arch-Angiogram
Information for patients Having an Aortic Arch-Angiogram Sheffield Teaching Hospitals Other names: Aortic arch-angiogram, arch-angiogram, arch-aortogram. You have been given this leaflet because you need a procedure known as an Aortic Arch-Angiogram. This leaflet explains more about Aortic Arch-Angiograms, and answers some of the most frequently asked questions. If, after reading this leaflet, you have any questions or concerns, you should write them down and discuss them at your next appointment with the consultant, doctor or specialist nurse. It is important that you understand the procedure, along with the potential benefits and risks before you agree to it. Where will my hospital appointments take place? This will depend on which specialist doctor you are seen by. You could be seen by a Neurologist, a Stroke Physician, a Vascular Surgeon or a Radiologist. Most of the appointments will be at either the Northern General or Royal Hallamshire Hospitals. However, you may also be seen at one of the outreach clinics at Rotherham or Barnsley District Hospitals. 2 What is an aortic arch-angiogram? An aortic arch-angiogram is an x-ray test that enables us to diagnose a problem (most commonly a narrowing or a blockage) in the arteries supplying your head, neck and arms. Arteries do not usually show up on x-rays, so the images are obtained by introducing a long, thin, flexible tube (a catheter) into an artery, usually at the top of your leg. Then, a special x-ray dye (contrast medium) is injected through it, into the circulation. The blood flow carries the dye along, highlighting the arteries, and x-ray pictures are taken. -
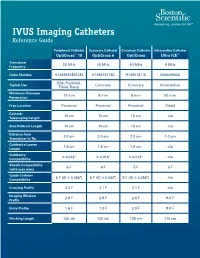
IVUS Imaging Catheters Reference Guide
IVUS Imaging Catheters Reference Guide Peripheral Catheter Coronary Catheter Coronary Catheter Intracardiac Catheter OptiCross™ 18 OptiCross 6 OptiCross Ultra ICE™ Transducer 30 MHz 40 MHz 40 MHz 9 MHz Frequency Order Number H7493932800180 H7495181160 H749518110 M00499000 SFA, Popliteal, Typical Use Coronary Coronary Intracardiac Tibial, Renal Maximum Diameter 12 mm 6 mm 6 mm 50 mm Penetration Prep Location Proximal Proximal Proximal Distal Catheter 15 cm 15 cm 15 cm n/a Telescoping Length Sled Pullback Length 10 cm 10 cm 10 cm n/a Distance from 2.0 cm 2.0 cm 2.0 cm 1.0 cm Transducer to Tip Guidewire Lumen 1.6 cm 1.6 cm 1.6 cm n/a Length Guidewire ≤ 0.018" ≤ 0.014" ≤ 0.014" n/a Compatibility Sheath Compatibility 6 F 6 F 5 F 9 F (with max wire) Guide Catheter 6 F (ID ≥ 0.068") 6 F (ID ≥ 0.064") 5 F (ID ≥ 0.058") n/a Compatibility Crossing Profile 3.5 F 3.1 F 3.1 F n/a Imaging Window 2.9 F 2.9 F 2.6 F 9.0 F Profile Entry Profile 1.6 F 1.3 F 2.0 F 9.0 F Working Length 135 cm 135 cm 135 cm 110 cm OPTICROSS™ 18 CATHETER AND MDU5 PLUS BAG OPTICROSS 6 40 MHZ CORONARY IMAGING CATHETER CAUTION Federal law (USA) restricts this device to sale by or on the order of a physician. Rx only. Prior to use, please see the CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician. -

Carotid Artery Stenting Initial Public Comments CAG-00085R June 18-July 18, 2004
Carotid Artery Stenting Initial Public Comments CAG-00085R June 18-July 18, 2004 Comment #1: Submitter: Peter Fail Organization: Cardiovascular Institute of the South Date: June 28, 2004 Comment: As an investigator of cartoid stenting in high risk patients, I feel that coverage of these patients will become a necesseity. The high risk patients not only has Carotid disease but usually a whole host of other vascular co- morbidiities that makes a surgical option "high risk". There are also those patients that the surgical option is non-existant due to anatomy weather a high or low lesions or because of prior radiation or surgery, etc. The proper training will difficult to access. Even those physicians in trial some of them have low numbers. (It is assumed that their numbers to get in to the trial was adequate). I am not sure what should be considered as an "adequate" number to be considered "trained". As the trials evolved the advent of embolic protection made the procedure "safer". There have been a number of times that I found debris in a filter and was thankful for it. The clinical event may not be that different with or without filters how ever I would argue that any debris in the brain is bad. It may not result in a clinically evident stroke, only a memory of a friend or something else that "can't" be tested for. I feel that using the current critera that were put forth as "high risk" by both SAPPHIRE and ARCHER some be atleast the baseline that can be used as a CAS requirement for "coverage". -

Intravascular Ultrasound and Magnetic Resonance Imaging Of
Intravascular Ultrasound and Magnetic Resonance Imaging of Atherosclerosis and Assessment of Endothelial Function Lachlan Frost Discipline of Medicine, School of Medicine The University of Adelaide & Cardiovascular Research Centre Royal Adelaide Hospital April 2015 Submitted in the total fulfilment of the requirements for the degree of Doctor of Philosophy i THESIS DECLARATION I certify that this work contains no material which has been accepted for the award of any other degree or diploma in any university or other tertiary institution and, to the best of my knowledge and belief, contains no material previously published or written by another person, except where due reference has been made in the text. In addition, I certify that no part of this work will, in the future, be used in a submission for any other degree or diploma in any university or other tertiary institution without the prior approval of the University of Adelaide and where applicable, any partner institution responsible for the joint-award of this degree. I give consent to this copy of my thesis when deposited in the University Library, being made available for loan and photocopying, subject to the provisions of the Copyright Act 1968. I also give permission for the digital version of my thesis to be made available on the web, via the University’s digital research repository, the Library Search and also through web search engines, unless permission has been granted by the University to restrict access for a period of time. Signed, Lachlan Frost University of Adelaide ii THESIS RELATED ABSTRACTS Frost L, Richardson J, Carbone A, Puri R, Nelson A, Sidhartha S, Worthley M, Worthley S. -

Investigating Signal Loss Due to a Carotid Artery Stent in 3D-TOF-MRA
Magn Reson Med Sci 2020; XX; XXX–XXX doi:10.2463/mrms.mp.2019-0083 Published Online: September 18, 2020 MAJOR PAPER Investigating Signal Loss due to a Carotid Artery Stent in 3D-TOF-MRA Hiroshi Kato*, Norio Ootani, Kentaro Abiru, and Mika Okahara Purpose: In this study, we investigated the factors of signal loss out because of the presence of a stent and optimized imaging parameters for improvement in depiction ability. Methods: We investigated the relationship between the stent type and magnetic susceptibility effect by mea- suring the signal value between the inside and outside of the stent with different Bw and TE for two different kinds of stents respectively. Similarly, flip angles were changed for two different kinds of stents respectively to the signal intensity between the inside and the outside of the stent was measured, in which examine the relationship between the stent type and the Ernst angles in RF-shielding effect. The conventional imaging parameters and the optimum imaging parameters for each stent obtained from the result of the phantom experiment were examined. Optimized 3D time-of-flight MR angiography (3D-TOF-MRA) was performed and compared with conventional 3D-TOF-MRA and computed tomography angiography (CTA). Results: The influence of the magnetic susceptibility effect is small in the central part of Carotid Wallstent and in PRECISE, and large in the Carotid Wallstent at the both ends. The influence of RF-shielding effect was large at PRECISE, where the Ernst angle was greatly shifted while the effect is no longer influenced at Carotid Wallstent. Both Carotid Wallstent and PRECISE made imaging capability improved by optimizing the imaging parameters. -

Intravascular Ultrasound for Coronary Vessels Policy Number: MP-091 Last Review Date: 11/14/2019 Effective Date: 01/01/2020
Intravascular Ultrasound for Coronary Vessels Policy Number: MP-091 Last Review Date: 11/14/2019 Effective Date: 01/01/2020 Policy Evolent Health considers Intravascular Ultrasound (IVUS) for Coronary Vessels medically necessary for either of the following indications: 1. IVUS of the coronary arteries (consistent with the 2011 ACCF/AHA Guidelines for Percutaneous Coronary Intervention (PCI) 5.4.2) is indicated for any of the following medical reasons: a. To confirm clinical suspicion of a significant left main coronary artery stenosis when standard angiography is indeterminate; b. To detect rapidly progressive cardiac allograft vasculopathy following heart transplant; c. To determine the mechanism of stent thrombosis or restenosis; d. To assess non-left main coronary arteries with angiographic intermediate stenosis (50-70%) to aid the decision whether or not to place a stent; or, e. To assist in guidance of complex coronary stent implementation, especially involving the L main coronary artery. 2. In lieu of coronary angiography when performed to minimize use of iodinated contrast material in an individual with compromised renal function, congestive heart failure or known contrast allergy. Limitations Coronary IVUS is not covered for any of the following (this is not an all-inclusive list): 1. Screening for coronary artery disease in asymptomatic individuals; 2. Routine lesion assessment is not recommended when revascularization with PCI or Coronary Artery Bypass Grafting (CABG) is not being considered; 3. Carotid stent placement; 4. Follow-up monitoring of medical therapies for atherosclerosis; 5. Peripheral vascular intervention; or, 6. Evaluation of chronic venous obstruction or to guide venous stenting. Background Ultrasound diagnostic procedures utilizing low energy sound waves are being widely employed to determine the composition and contours of nearly all body tissues except bone and air-filled spaces. -

Clinical Guideline Optical Coherence Tomography (OCT)
Clinical Guideline Guideline Number: CG025, Ver. 2 Optical Coherence Tomography (OCT) Disclaimer Clinical guidelines are developed and adopted to establish evidence-based clinical criteria for utilization management decisions. Oscar may delegate utilization management decisions of certain services to third-party delegates, who may develop and adopt their own clinical criteria. The clinical guidelines are applicable to all commercial plans. Services are subject to the terms, conditions, limitations of a member’s plan contracts, state laws, and federal laws. Please reference the member’s plan contracts (e.g., Certificate/Evidence of Coverage, Summary/Schedule of Benefits) or contact Oscar at 855-672-2755 to confirm coverage and benefit conditions. Summary Optical Coherence Tomography, or “OCT”, is a medical imaging test that uses light waves to capture live 3-dimensional images. It is similar in principle to ultrasound (which uses sound echoes, rather than light wave reflections), however OCT provides up to 10 times the resolution. OCT has been used to image different structures of the body, including the eye, the heart, the gastrointestinal (GI) system, the breast, and the upper airway. It does not require any contact with the target surfaces and does not produce any ionizing radiation. In some cases, OCT can be used with other instruments such as an endoscope in the GI system or as an intravascular device in the arteries of the heart. OCT is a relatively novel technology and is rapidly evolving in both technique and clinical utility. This guideline provides the clinical criteria and exclusions for the currently supported clinical applications of Optical Coherence Tomography. -

Carotid Endarterectomy Compared with Angioplasty and Stenting: the Status of the Debate
Neurosurg Focus 5 (6):Article 2, 1998 Carotid endarterectomy compared with angioplasty and stenting: the status of the debate Felipe C. Albuquerque, M.D., George P. Teitelbaum, M.D., Donald W. Larsen, M.D., and Steven L. Giannotta, M.D. Department of Neurological Surgery, Los Angeles County and University of Southern California Medical Center, Los Angeles, California Endarterectomy is the treatment of choice for patients with symptomatic stenosis of the internal carotid artery. Recently, debate has arisen over the potential benefits of endovascular techniques. Although retrospective analyses of angioplasty and stenting procedures suggest comparable clinical efficacy to endarterectomy, prospective evaluation is pending. The authors review the status of the debate and discuss those issues on both sides that are particularly contentious and clinically relevant. Key Words * carotid endarterectomy * angioplasty * stenting Atherosclerotic disease of the common carotid artery bifurcation is associated with 20 to 30% of cerebrovascular accidents.[13,15,27] Stroke is the third leading cause of death in the United States and the most common and disabling neurological disorder among the elderly worldwide.[13,15,27] In light of these public health concerns, research in the last half of this century has been focused on the optimum treatment of carotid artery stenosis. Prospective analyses such as those performed by the North American Symptomatic Carotid Endarterectomy Trial (NASCET), the Asymptomatic Carotid Atherosclerosis Study (ACAS), and the European Carotid Surgery Trial have demonstrated superior reduction in stroke incidence among symptomatic and a select group of asymptomatic patients who undergo carotid endarterectomy (CEA).[17,18,36] In fact, these studies have established CEA as the "gold standard" for the treatment of carotid artery atherosclerosis. -

Final Addenda FY 2005
FY 2005 Final Addenda ICD-9-CM Volume 3, Procedures Effective October 1, 2004 Tabular List 00.0 Therapeutic ultrasound Add exclusion term Excludes: diagnostic ultrasound (non-invasive) (88.71-88.79) intracardiac echocardiography [ICE] (heart chamber(s)) (37.28) intravascular imaging (adjunctive) (00.21-00.29) New code 00.16 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Ex-vivo treatment of vessel Hyperbaric pressurized graft [conduit] New code 00.17 Infusion of vasopressor agent New subcategory 00.2 Intravascular imaging of blood vessels Endovascular ultrasonography Intravascular ultrasound (IVUS) Code also any synchronous diagnostic or therapeutic procedures Excludes: therapeutic ultrasound (00.01-00.09) New code 00.21 Intravascular imaging of extracranial cerebral vessels Common carotid vessels and branches Intravascular ultrasound (IVUS), extracranial cerebral vessels Excludes: diagnostic ultrasound (non-invasive) of head and neck (88.71) New code 00.22 Intravascular imaging of intrathoracic vessels Aorta and aortic arch Intravascular ultrasound (IVUS), intrathoracic vessels Vena cava (superior) (inferior) Excludes: diagnostic ultrasound (non-invasive) of other sites of thorax (88.73) New code 00.23 Intravascular imaging of peripheral vessels Imaging of: vessels of arm(s) vessels of leg(s) Intravascular ultrasound (IVUS), peripheral vessels Excludes: diagnostic ultrasound (non-invasive) of peripheral vascular system (88.77) New code 00.24 Intravascular imaging of coronary vessels Intravascular -

Intravascular Ultrasound Appearance of Normal and Mildly Diseased Coronary Arteries: Correlation with Histologic Specimens
Intravascular ultrasound appearance of normal and mildly diseased coronary arteries: Correlation with histologic specimens Bavani Maheswaran, MD, Cyril Y. Leung, MD, Dan E. Gutfinger, PhD, Shigeru Nakamura, MD, Robert J. Russo, MD, Takafumi Hire, MD, and Jonathan M. Tobis, MD Irvine, Calif. Intravascular ultrasound imaging was performed in vitro on vestigate the histologic tissue characteristics that six histologically normal and 104 minimally diseased art- determine the ultrasound representation of coronary eries in patients aged 13 to 83 years. This study tested the artery microanatomy. hypothesis that normal coronary arteries produce a three- layer image that corresponds to the histologic layers of in- METHODS tima, media, and adventitia. The results showed a very good Human coronary arteries, Coronary artery segments correlation between area of the echolucent ultrasound layer with the media and the inner echogenic layer with intimal that appeared grossly normal by palpation were obtained area. In addition, a three-layer appearance was consistently at autopsy from 110 people over a wide age range. Because seen when the internal elastic membrane was present with the arteries were obtained from the coroner's office, little or without intimal hyperplasia. If the internal elastic mem- other clinical information was available. At the time of ul- brane was absent, a three-layer appearance was still seen if trasound imaging, the arteries were fresh and tmY-Lxed.An the collagen content of the media was low. However, a two- 8F guiding catheter was inserted into the proximal end of layer appearance was observed when there was absence of the vessel, and normal saline was perfused at 100 mm Hg the internal elastic membrane as well as a high collagen at room temperature in a saline bath. -

Current State-Of-The-Art in Intravascular Coronary Imaging
Current Cardiovascular Imaging Reports (2019) 12: 29 https://doi.org/10.1007/s12410-019-9503-7 INTRAVASCULAR IMAGING (A TRUESDELL, SECTION EDITOR) IVUS and OCT: Current State-of-the-Art in Intravascular Coronary Imaging Michael F. Bode1 & Farouc A. Jaffer1 Published online: 31 May 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Purpose of Review To summarize the current state-of-the-art of intracoronary imaging with a focus on clinical outcomes, novel approaches, and anticipated future developments. Recent Findings Multiple randomized trials have demonstrated that intravascular ultrasound (IVUS) guidance of percutaneous coronary intervention (PCI) significantly reduces major cardiac adverse events (MACE), and in particular, rates of target lesion failure or clinical restenosis. High-definition IVUS enhances the ability to visualize stent struts and plaque components. Optical coherence tomography (OCT) offers a greater resolution and can image many plaque morphology and stent characteristics that are not visualized with IVUS. Image guidance should be considered strongly in complex PCI lesions and potentially in all- comers. Multimodality imaging using near-infrared spectroscopy or fluorescence, combined with IVUS or OCT, is an exciting new approache to image plaque lipid, intraplaque hemorrhage, inflammation, and fibrin deposition. Summary Intravascular imaging guidance of PCI utilizing IVUS or OCT definitively improves outcomes in many patient subsets but remains heavily underutilized. Emerging intravascular imaging modalities, such as near-infrared spectroscopy and near- infrared fluorescence imaging, will allow for improved plaque and stent characterization. As cath lab integration, automated image segmentation, and x-ray co-registration evolve further, we envision that intravascular imaging will become a routine for the vast majority of PCI patients, with resultant benefits in clinical outcomes.