SI Handbook for the Lawrence Livermore National Laboratory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Representation of Derived Units in Unitsml (Revised December 8, 2006)
Representation of Derived Units in UnitsML (revised December 8, 2006) Peter J. Linstrom∗ December 8, 2006 ∗phone: (301) 975-5422,DRAFT e-mail: [email protected] 1 INTRODUCTION 12/8/06 Contents 1 Introduction 1 2 Why this convention is needed 2 3 Information needed to define a unit 3 4 Proposed XML encoding 4 5 Important conventions 7 6 Potential problems 8 7 Possible alternatives 10 A Multiplicative prefixes 12 B SI units and units acceptable for use with the SI 14 C non-SI Units 19 1 Introduction This document describes a proposed convention for defining derived units in terms of their base units. This convention is intended for use in the UnitsML markup language to allow a precise definition of a wide range of units. The goal of this convention is to improve interoperability among applications and databases which use derived units based on commonly encountered base units. It is understoodDRAFT that not all units can be represented using this convention. It is, however, Representation of Derived Units in UnitsML (revised December 8, 2006) Page 1 2 WHY THIS CONVENTION IS NEEDED 12/8/06 anticipated that a wide range of scientific and engineering units of measure can be represented with this convention. The convention consists of representing the unit in terms of multiplicative combinations of base units. For example the unit centimeter per second squared would be represented in terms of the following: 1. The unit meter with the prefix centi raised to the power 1. 2. The unit second raised to the power −2. -

Astm Metric Practice Guide
National Bureau of Standards Library, E-01 Admrn. Bldg. A111DO cmfl4S - m 2 0 1967 METRIC PRACTICE GUIDE NATL INST OF STANDARDS & TECH R.I.C. All100991845 /NBS handbook QC1 .U51 V102;1967 C.1 NBS-PUB-C 1934 U. S. DEPARTMENT OF COMMERCE NATIONAL OUREAU OF STANDARDS HANDBOOK 102 THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards 1 provides measurement and technical information services essential to the efficiency and effectiveness of the work of the Nation’s scientists and engineers. The Bureau serves also as a focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To accomplish this mission, the Bureau is organized into three institutes covering broad program areas of research and services: THE INSTITUTE FOR BASIC STANDARDS . provides the central basis within the United States for a complete and consistent system of physical measurements, coordinates that system with the measurement systems of other nations, and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scientific com¬ munity, industry, and commerce. This Institute comprises a series of divisions, each serving a classical subject matter area: —Applied Mathematics — Electricity—Metrology — Mechanics — Heat — Atomic Physics—Physical Chemistry—Radiation Physics—Laboratory Astrophysics2—Radio Standards Laboratory,2 which includes Radio Standards Physics and Radio Standards Engineering—Office of Standard Reference Data. THE INSTITUTE FOR MATERIALS RESEARCH . conducts materials research and provides associated materials services including mainly reference materials and data on the properties of materials. Beyond its direct interest to the Nation’s scientists and engineers, this Institute yields services which are essential to the advancement of technology in industry and commerce. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -
Manual of Style
Manual Of Style 1 MANUAL OF STYLE TABLE OF CONTENTS CHAPTER 1 GENERAL PROVISIONS ...............3 101.0 Scope .............................................. 3 102.0 Codes and Standards..................... 3 103.0 Code Division.................................. 3 104.0 Table of Contents ........................... 4 CHAPTER 2 ADMINISTRATION .........................6 201.0 Administration ................................. 6 202.0 Chapter 2 Definitions ...................... 6 203.0 Referenced Standards Table ......... 6 204.0 Individual Chapter Administrative Text ......................... 6 205.0 Appendices ..................................... 7 206.0 Installation Standards ..................... 7 207.0 Extract Guidelines .......................... 7 208.0 Index ............................................... 9 CHAPTER 3 TECHNICAL STYLE .................... 10 301.0 Technical Style ............................. 10 302.0 Technical Rules ............................ 10 Table 302.3 Possible Unenforceable and Vague Terms ................................ 10 303.0 Health and Safety ........................ 11 304.0 Rules for Mandatory Documents .................................... 11 305.0 Writing Mandatory Requirements ............................... 12 Table 305.0 Typical Mandatory Terms............. 12 CHAPTER 4 EDITORIAL STYLE ..................... 14 401.0 Editorial Style ................................ 14 402.0 Definitions ...................................... 14 403.0 Units of Measure ............................ 15 404.0 Punctuation -

CAR-ANS Part 5 Governing Units of Measurement to Be Used in Air and Ground Operations
CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES Part 5 Governing UNITS OF MEASUREMENT TO BE USED IN AIR AND GROUND OPERATIONS CIVIL AVIATION AUTHORITY OF THE PHILIPPINES Old MIA Road, Pasay City1301 Metro Manila UNCOTROLLED COPY INTENTIONALLY LEFT BLANK UNCOTROLLED COPY CAR-ANS PART 5 Republic of the Philippines CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES (CAR-ANS) Part 5 UNITS OF MEASUREMENTS TO BE USED IN AIR AND GROUND OPERATIONS 22 APRIL 2016 EFFECTIVITY Part 5 of the Civil Aviation Regulations-Air Navigation Services are issued under the authority of Republic Act 9497 and shall take effect upon approval of the Board of Directors of the CAAP. APPROVED BY: LT GEN WILLIAM K HOTCHKISS III AFP (RET) DATE Director General Civil Aviation Authority of the Philippines Issue 2 15-i 16 May 2016 UNCOTROLLED COPY CAR-ANS PART 5 FOREWORD This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was formulated and issued by the Civil Aviation Authority of the Philippines (CAAP), prescribing the standards and recommended practices for units of measurements to be used in air and ground operations within the territory of the Republic of the Philippines. This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was developed based on the Standards and Recommended Practices prescribed by the International Civil Aviation Organization (ICAO) as contained in Annex 5 which was first adopted by the council on 16 April 1948 pursuant to the provisions of Article 37 of the Convention of International Civil Aviation (Chicago 1944), and consequently became applicable on 1 January 1949. The provisions contained herein are issued by authority of the Director General of the Civil Aviation Authority of the Philippines and will be complied with by all concerned. -

CAR-ANS PART 05 Issue No. 2 Units of Measurement to Be Used In
CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES Part 5 Governing UNITS OF MEASUREMENT TO BE USED IN AIR AND GROUND OPERATIONS CIVIL AVIATION AUTHORITY OF THE PHILIPPINES Old MIA Road, Pasay City1301 Metro Manila INTENTIONALLY LEFT BLANK CAR-ANS PART 5 Republic of the Philippines CIVIL AVIATION REGULATIONS AIR NAVIGATION SERVICES (CAR-ANS) Part 5 UNITS OF MEASUREMENTS TO BE USED IN AIR AND GROUND OPERATIONS 22 APRIL 2016 EFFECTIVITY Part 5 of the Civil Aviation Regulations-Air Navigation Services are issued under the authority of Republic Act 9497 and shall take effect upon approval of the Board of Directors of the CAAP. APPROVED BY: LT GEN WILLIAM K HOTCHKISS III AFP (RET) DATE Director General Civil Aviation Authority of the Philippines Issue 2 15-i 16 May 2016 CAR-ANS PART 5 FOREWORD This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was formulated and issued by the Civil Aviation Authority of the Philippines (CAAP), prescribing the standards and recommended practices for units of measurements to be used in air and ground operations within the territory of the Republic of the Philippines. This Civil Aviation Regulations-Air Navigation Services (CAR-ANS) Part 5 was developed based on the Standards and Recommended Practices prescribed by the International Civil Aviation Organization (ICAO) as contained in Annex 5 which was first adopted by the council on 16 April 1948 pursuant to the provisions of Article 37 of the Convention of International Civil Aviation (Chicago 1944), and consequently became applicable on 1 January 1949. The provisions contained herein are issued by authority of the Director General of the Civil Aviation Authority of the Philippines and will be complied with by all concerned. -

Package 'Nistunits'
Package ‘NISTunits’ August 11, 2016 Type Package Title Fundamental Physical Constants and Unit Conversions from NIST Version 1.0.1 Encoding UTF-8 Description Fundamental physical constants (Quantity, Value, Uncertainty, Unit) for SI (International System of Units) and non-SI units, plus unit conversions Based on the data from NIST (National Institute of Standards and Technology, USA) License GPL (>= 3) Depends R (>= 2.7.0) Author Jose Gama [aut, cre] Maintainer Jose Gama <[email protected]> Repository CRAN Repository/R-Forge/Project nistunits Repository/R-Forge/Revision 4 Repository/R-Forge/DateTimeStamp 2016-08-11 06:51:12 Date/Publication 2016-08-11 13:47:23 NeedsCompilation no R topics documented: CompactFactorNames . 20 CompactPhysicalConstantNames . 21 FixFactorNames . 22 kNIST2010latticeSpacingOfSilicon . 22 kNISTnonSIatomicUnitOf1stHyperpolarizability . 33 NISTabampereTOampere . 34 NISTabcoulombTOcoulomb . 35 NISTabfaradTOfarad . 36 NISTabhenryTOhenry . 37 NISTabmhoTOsiemens . 38 NISTabohmTOohm . 39 1 2 R topics documented: NISTabvoltTOvolt . 40 NISTaccelOfFreeFallStdTOmeterPerSecSqrd . 41 NISTacreFtTOcubMeter . 42 NISTacreTOsqrMeter . 43 NISTampereHourTOcoulomb . 44 NISTamperePerMeterTOoersted . 45 NISTampereTOabampere . 46 NISTampereTObiot . 47 NISTampereToEMUOfCurrent . 48 NISTampereToESUOfCurrent . 49 NISTampereTOgilbert . 50 NISTampereTOstatampere . 51 NISTangstromTOmeter . 52 NISTangstromTOnanometer . 53 NISTareTOsqrMeter . 54 NISTastronomicalUnTOmeter . 55 NISTatmosphereStdTOkpascal . 56 NISTatmosphereStdTOpascal . 57 -
Tableau De Conversion D'unités,NL8.4FT
Tableau de conversion d’unités 1. 2. 3. Tableau de conversion d’unités Acceleration foot/second2, meter/second2, gal, galileo, inch/second2 1 m/s2 = 3.28084 ft/s2 = 100 cm/s2 = 39.37 inch per second squared (inch/s2) 1 ft/s2 = 0.3048 m/s2 = 30.48 cm/s2 1 g = 9.80665 m/s2 = 32.17405 ft/s2 Angle 1 circle = 360 degrees = 400 grades = 21600 minutes = 6.28318 radians = 12 signs 1 circumference = 360 degrees = 6.28318 radians 1 radian = 0.15915 circumference = 57.29578 degree = 3437.747 minute = 0.63662 quadrant = 0.15915 revolution = 206265 second Area acre, are, barn, sq.ft., sq.in., foot2, hectare, inch2, mile2, section, meter2, township, yard2, hectares 1 m2 = 1550 in2 = 10.764 ft2 = 1.1968 yd2 = 3.861×10-7 mile2 1 ft2 = 0.0929 m2 = 144 in2 = 0,1111 yd2 = 3.587×10-8 mile2 1 in2 = 6.452 cm2 = 6.452×10-4 m2 = 6.944×10-3 ft2 = 7.716×10-4 yd2 = 2.491×10-10mile2 1 yd2 = 0.8361 m2 = 1,296 in2 = 9 ft2 = 0.3228×10-6 mile2 1 mile2 = 2.590×106 m2 = 0.4015×1010 in2 = 2.788×107 ft2 = 3.098×106 yd2=640 Acres 1 acre = 1/640 square mile = 0.404686 ha (Hectares) = 4,046.86 m2 = 43,560.174 Sq.Ft. (Int) = 43,560 Sq.Ft. (US Survey) = 4840 Sq.Yds. = 40.46873 are 1 km2 = 102 ha2 = 106 m2 = 1010 cm2 = 1012 mm2 1 ha (Hectare) = 104 m2 = 108 cm2 = 1010 mm2 = 2.471 Acres 1 cm2 = 10-4 m2 = 0.155 in2 1 mm2 = 1.55×10-3in2 1 township = 36 square mile = 23040 acre = 36 section = 9.323957 107 m2 = 9324 hectare = 93.24 square kilometer 1 section = 1 square mile = 2.59 106 m2 = 2.59 square kilometer = 259 hectare = 3.0976 106 square yards = 640 acre = 1 are = 0.024711 acre -

Units of Measurement to Be Used in Air and Ground Operations
International Standards and Recommended Practices Annex 5 to the Convention on International Civil Aviation Units of Measurement to be Used in Air and Ground Operations This edition incorporates all amendments adopted by the Council prior to 23 February 2010 and supersedes, on 18 November 2010, all previous editions of Annex 5. For information regarding the applicability of the Standards and Recommended Practices,see Foreword. Fifth Edition July 2010 International Civil Aviation Organization Suzanne TRANSMITTAL NOTE NEW EDITIONS OF ANNEXES TO THE CONVENTION ON INTERNATIONAL CIVIL AVIATION It has come to our attention that when a new edition of an Annex is published, users have been discarding, along with the previous edition of the Annex, the Supplement to the previous edition. Please note that the Supplement to the previous edition should be retained until a new Supplement is issued. Suzanne International Standards and Recommended Practices Annex 5 to the Convention on International Civil Aviation Units of Measurement to be Used in Air and Ground Operations ________________________________ This edition incorporates all amendments adopted by the Council prior to 23 February 2010 and supersedes, on 18 November 2010, all previous editions of Annex 5. For information regarding the applicability of the Standards and Recommended Practices, see Foreword. Fifth Edition July 2010 International Civil Aviation Organization Published in separate English, Arabic, Chinese, French, Russian and Spanish editions by the INTERNATIONAL CIVIL AVIATION ORGANIZATION 999 University Street, Montréal, Quebec, Canada H3C 5H7 For ordering information and for a complete listing of sales agents and booksellers, please go to the ICAO website at www.icao.int First edition 1948 Fourth edition 1979 Fifth edition 2010 Annex 5, Units of Measurement to be Used in Air and Ground Operations Order Number: AN 5 ISBN 978-92-9231-512-2 © ICAO 2010 All rights reserved. -
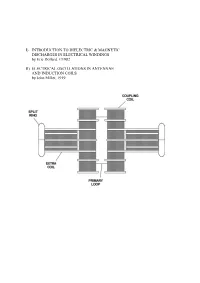
I) Introduction to Dielectric & Magnetic Discharges In
I) INTRODUCTION TO DIELECTRIC & MAGNETIC DISCHARGES IN ELECTRICAL WINDINGS by Eric Dollard, ©1982 II) ELECTRICAL OSCILLATIONS IN ANTENNAE AND INDUCTION COILS by John Miller, 1919 PART I INTRODUCTION TO DIELECTRIC & MAGNETIC DISCHARGES IN ELECTRICAL WINDINGS by Eric Dollard, ©1982 1. CAPACITANCE 2. CAPACITANCE INADEQUATELY EXPLAINED 3. LINES OF FORCE AS REPRESENTATION OF DIELECTRICITY 4. THE LAWS OF LINES OF FORCE 5. FARADAY'S LINES OF FORCE THEORY 6. PHYSICAL CHARACTERISTICS OF LINES OF FORCE 7. MASS ASSOCIATED WITH LINES OF FORCE IN MOTION 8. INDUCTANCE AS AN ANALOGY TO CAPACITANCE 9. MECHANISM OF STORING ENERGY MAGNETICALLY 10. THE LIMITS OF ZERO AND INFINITY 11. INSTANT ENERGY RELEASE AS INFINITY 12. ANOTHER FORM OF ENERGY APPEARS 13. ENERGY STORAGE SPATIALLY DIFFERENT THAN MAGNETIC ENERGY STORAGE 14. VOTAGE IS TO DIELECTRICITY AS CURRENT IS TO MAGNETISM 15. AGAIN THE LIMITS OF ZERO AND INFINITY 16. INSTANT ENERGY RELEASE AS INFINITY 17. ENERGY RETURNS TO MAGNETIC FORM 18. CHARACTERISTIC IMPEDANCE AS A REPRESENTATION OF PULSATION OF ENERGY 19. ENERGY INTO MATTER 20. MISCONCEPTION OF PRESENT THEORY OF CAPACITANCE 21. FREE SPACE INDUCTANCE IS INFINITE 22. WORK OF TESLA, STEINMETZ, AND FARADAY 23. QUESTION AS TO THE VELOCITY OF DIELECTRIC FLUX APPENDIX I 0) Table of Units, Symbols & Dimensions 1) Table of Magnetic & Dielectric Relations 2) Table of Magnetic, Dielectric & Electronic Relations PART II ELECTRICAL OSCILLATIONS IN ANTENNAE & INDUCTION COILS J.M. Miller Proceedings, Institute of Radio Engineers. 1919 1) CAPACITANCE The phenomena of capacitance is a type of electrical energy storage in the form of a field in an enclosed space. This space is typically bounded by two parallel metallic plates or two metallic foils on an interviening insulator or dielectric. -

Chemenghbk-Ch01-Conversion-Factors.Pdf
Copyright © 2008, 1997, 1984, 1973, 1963, 1950, 1941, 1934 by The McGraw-Hill Companies, Inc. All rights reserved. Manufactured in the United States of America. Except as permitted under the United States Copyright Act of 1976, no part of this publication may be reproduced or distributed in any form or by any means, or stored in a database or retrieval system, without the prior written permission of the publisher. 0-07-154208-6 The material in this eBook also appears in the print version of this title: 0-07-151124-5. All trademarks are trademarks of their respective owners. Rather than put a trademark symbol after every occurrence of a trademarked name, we use names in an editorial fashion only, and to the benefit of the trademark owner, with no intention of infringement of the trademark. Where such desig- nations appear in this book, they have been printed with initial caps. McGraw-Hill eBooks are available at special quantity discounts to use as premiums and sales promotions, or for use in corporate training programs. For more information, please contact George Hoare, Special Sales, at [email protected] or (212) 904-4069. TERMS OF USE This is a copyrighted work and The McGraw-Hill Companies, Inc. (“McGraw-Hill”) and its licensors reserve all rights in and to the work. Use of this work is subject to these terms. Except as permitted under the Copyright Act of 1976 and the right to store and retrieve one copy of the work, you may not decompile, disassemble, reverse engineer, reproduce, modify, create derivative works based upon, transmit, distribute, disseminate, sell, publish or sublicense the work or any part of it without McGraw-Hill’s prior consent. -

Here Is How Ithkuil Handles Mathematical Expressions and Units
EXPRESSING MATHEMATICS AND MEASUREMENT IN ITHKUIL I have determined that, using existing Ithkuil morpho-phonology, morphology and morpho-syntax, there really isn’t an efficient way to state mathematical expressions in Ithkuil, including mathematical expressions involving units of measurement. Therefore, I’ve decided to create a formal sub-language within Ithkuil for dealing with mathematical expressions and units of measurement. You can think of this as a verbal analogy to the way that real-world written forms of mathematical expressions and rates of measurement require their own formal set of written symbols and symbolic notation rather than writing out mathematical expressions in words. Note that I have chosen to maintain the existing informal centesimal system without a root for zero as described in Chapter 12 of the Ithkuil website, as a means for efficiently conveying an everyday “naïve” means of doing counting and very basic arithmetical operations consistent with the morpho-phonology, morphology, and morpho-syntax of the Ithkuil language. At the same time, having a formal sub-language for higher mathematical expressions and measurement that follows its own internal morphological and syntactical rules allows for a succinct means of verbal mathematical expression and underscores the formalized, “special case” nature of mathematical expressions, again analagous to the formal written notation for mathematical expressions in real-world languages. This treatise is in two parts; the first part focusing on mathematical expressions, the second part on units of mearurement. MATHEMATICAL EXPRESSIONS Before introducing the new Ithkuil sub-language for formal mathematical expressions and measurement, I will first introduce the new Ithkuil roots, stems, and suffixes necessary for referencing higher mathematical concepts, terminology and expressions.