Gene Expression Biomarkers and Longevity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table 2. Functional Classification of Genes Differentially Regulated After HOXB4 Inactivation in HSC/Hpcs
Table 2. Functional classification of genes differentially regulated after HOXB4 inactivation in HSC/HPCs Symbol Gene description Fold-change (mean ± SD) Signal transduction Adam8 A disintegrin and metalloprotease domain 8 1.91 ± 0.51 Arl4 ADP-ribosylation factor-like 4 - 1.80 ± 0.40 Dusp6 Dual specificity phosphatase 6 (Mkp3) - 2.30 ± 0.46 Ksr1 Kinase suppressor of ras 1 1.92 ± 0.42 Lyst Lysosomal trafficking regulator 1.89 ± 0.34 Mapk1ip1 Mitogen activated protein kinase 1 interacting protein 1 1.84 ± 0.22 Narf* Nuclear prelamin A recognition factor 2.12 ± 0.04 Plekha2 Pleckstrin homology domain-containing. family A. (phosphoinosite 2.15 ± 0.22 binding specific) member 2 Ptp4a2 Protein tyrosine phosphatase 4a2 - 2.04 ± 0.94 Rasa2* RAS p21 activator protein 2 - 2.80 ± 0.13 Rassf4 RAS association (RalGDS/AF-6) domain family 4 3.44 ± 2.56 Rgs18 Regulator of G-protein signaling - 1.93 ± 0.57 Rrad Ras-related associated with diabetes 1.81 ± 0.73 Sh3kbp1 SH3 domain kinase bindings protein 1 - 2.19 ± 0.53 Senp2 SUMO/sentrin specific protease 2 - 1.97 ± 0.49 Socs2 Suppressor of cytokine signaling 2 - 2.82 ± 0.85 Socs5 Suppressor of cytokine signaling 5 2.13 ± 0.08 Socs6 Suppressor of cytokine signaling 6 - 2.18 ± 0.38 Spry1 Sprouty 1 - 2.69 ± 0.19 Sos1 Son of sevenless homolog 1 (Drosophila) 2.16 ± 0.71 Ywhag 3-monooxygenase/tryptophan 5- monooxygenase activation protein. - 2.37 ± 1.42 gamma polypeptide Zfyve21 Zinc finger. FYVE domain containing 21 1.93 ± 0.57 Ligands and receptors Bambi BMP and activin membrane-bound inhibitor - 2.94 ± 0.62 -

Genomic Correlates of Relationship QTL Involved in Fore- Versus Hind Limb Divergence in Mice
Loyola University Chicago Loyola eCommons Biology: Faculty Publications and Other Works Faculty Publications 2013 Genomic Correlates of Relationship QTL Involved in Fore- Versus Hind Limb Divergence in Mice Mihaela Palicev Gunter P. Wagner James P. Noonan Benedikt Hallgrimsson James M. Cheverud Loyola University Chicago, [email protected] Follow this and additional works at: https://ecommons.luc.edu/biology_facpubs Part of the Biology Commons Recommended Citation Palicev, M, GP Wagner, JP Noonan, B Hallgrimsson, and JM Cheverud. "Genomic Correlates of Relationship QTL Involved in Fore- Versus Hind Limb Divergence in Mice." Genome Biology and Evolution 5(10), 2013. This Article is brought to you for free and open access by the Faculty Publications at Loyola eCommons. It has been accepted for inclusion in Biology: Faculty Publications and Other Works by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. © Palicev et al., 2013. GBE Genomic Correlates of Relationship QTL Involved in Fore- versus Hind Limb Divergence in Mice Mihaela Pavlicev1,2,*, Gu¨ nter P. Wagner3, James P. Noonan4, Benedikt Hallgrı´msson5,and James M. Cheverud6 1Konrad Lorenz Institute for Evolution and Cognition Research, Altenberg, Austria 2Department of Pediatrics, Cincinnati Children‘s Hospital Medical Center, Cincinnati, Ohio 3Yale Systems Biology Institute and Department of Ecology and Evolutionary Biology, Yale University 4Department of Genetics, Yale University School of Medicine 5Department of Cell Biology and Anatomy, The McCaig Institute for Bone and Joint Health and the Alberta Children’s Hospital Research Institute for Child and Maternal Health, University of Calgary, Calgary, Canada 6Department of Anatomy and Neurobiology, Washington University *Corresponding author: E-mail: [email protected]. -

Functional Analysis of Human Hematopoietic Stem Cell Gene Expression Using Zebrafish
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central Open access, freely available online PLoS BIOLOGY Functional Analysis of Human Hematopoietic Stem Cell Gene Expression Using Zebrafish Craig E. Eckfeldt1[, Eric M. Mendenhall1[, Catherine M. Flynn1, Tzu-Fei Wang1, Michael A. Pickart2, Suzanne M. Grindle3, Stephen C. Ekker2, Catherine M. Verfaillie1* 1 Department of Medicine, Division of Hematology, Oncology, and Transplantation, and Stem Cell Institute, University of Minnesota, Minneapolis, Minnesota, United States of America, 2 Genetics, Cell Biology, and Development and Arnold and Mabel Beckman Center for Transposon Research, University of Minnesota, Minneapolis, Minnesota, United States of America, 3 Cancer Center Bioinformatics Division, University of Minnesota, Minneapolis, Minnesota, United States of America Although several reports have characterized the hematopoietic stem cell (HSC) transcriptome, the roles of HSC-specific genes in hematopoiesis remain elusive. To identify candidate regulators of HSC fate decisions, we compared the transcriptome of human umbilical cord blood and bone marrow CD34þCD33ÀCD38ÀRholoc-kitþ cells, enriched for hematopoietic stem/progenitor cells with CD34þCD33ÀCD38ÀRhohi cells, enriched in committed progenitors. We identified 277 differentially expressed transcripts conserved in these ontogenically distinct cell sources. We next performed a morpholino antisense oligonucleotide (MO)-based functional screen in zebrafish to determine the hematopoietic function of 61 genes that had no previously known function in HSC biology and for which a likely zebrafish ortholog could be identified. MO knock down of 14/61 (23%) of the differentially expressed transcripts resulted in hematopoietic defects in developing zebrafish embryos, as demonstrated by altered levels of circulating blood cells at 30 and 48 h postfertilization and subsequently confirmed by quantitative RT-PCR for erythroid-specific hbae1 and myeloid-specific lcp1 transcripts. -

A Conserved Gene Family Encodes Transmembrane Proteins with Fibronectin, Immunoglobulin and Leucine-Rich Repeat Domains (FIGLER) Delicia L
A conserved gene family encodes transmembrane proteins with fibronectin, immunoglobulin and leucine-rich repeat domains (FIGLER) Delicia L. Munfus, University of Alabama at Birmingham Christopher L. Haga, University of Alabama at Birmingham Peter D. Burrows, University of Alabama at Birmingham Max Cooper, Emory University Journal Title: BMC Biology Volume: Volume 5, Number 1 Publisher: BioMed Central | 2007-09-13, Pages 36-36 Type of Work: Article | Final Publisher PDF Publisher DOI: 10.1186/1741-7007-5-36 Permanent URL: https://pid.emory.edu/ark:/25593/rqdhh Final published version: http://dx.doi.org/10.1186/1741-7007-5-36 Copyright information: © 2007 Munfus et al; licensee BioMed Central Ltd. This is an Open Access work distributed under the terms of the Creative Commons Attribution 2.0 Generic License (http://creativecommons.org/licenses/by/2.0/). Accessed October 6, 2021 6:19 AM EDT BMC Biology BioMed Central Research article Open Access A conserved gene family encodes transmembrane proteins with fibronectin, immunoglobulin and leucine-rich repeat domains (FIGLER) Delicia L Munfus†1,2, Christopher L Haga†1,2, Peter D Burrows1,2,3 and Max D Cooper*1,2,4,5 Address: 1Division of Developmental and Clinical Immunology, University of Alabama at Birmingham, Birmingham, AL 35294-3300, USA, 2Department of Microbiology, University of Alabama at Birmingham, Birmingham, AL 35294-3300, USA, 3Department of Genetics, University of Alabama at Birmingham, Birmingham, AL 35294-3300,, 4Department of Medicine, University of Alabama at Birmingham, Birmingham, -

Tyrosine Kinase Oncogenes in Normal Hematopoiesis and Hematological Disease
Oncogene (2002) 21, 3314 ± 3333 ã 2002 Nature Publishing Group All rights reserved 0950 ± 9232/02 $25.00 www.nature.com/onc Tyrosine kinase oncogenes in normal hematopoiesis and hematological disease Blanca Scheijen1,2 and James D Grin*,1,2 1Department of Adult Oncology, Dana-Farber Cancer Institute, 44 Binney Street, Boston, Massachusetts, MA 02115, USA; 2Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA Tyrosine kinase oncogenes are formed as a result of Adaptors do not contain intrinsic catalytic activity but mutations that induce constitutive kinase activity. Many consist of independent functioning interaction modules of these tyrosine kinase oncogenes that are derived from like SH2-domain (mediates binding to phosphotyrosine genes, such as c-Abl, c-Fes, Flt3, c-Fms, c-Kit and residues), SH3-domain (interacts with polyproline-rich PDGFRb, that are normally involved in the regulation of PXXP stretch) or pleckstrin homology (PH) domain hematopoiesis or hematopoietic cell function. Despite (binds to inositol lipids). dierences in structure, normal function, and subcellular The ®rst tyrosine kinase oncogene associated with location, many of the tyrosine kinase oncogenes signal human hematologic disease, Bcr ± Abl, was identi®ed through the same pathways, and typically enhance almost twenty years ago, and there is now evidence for proliferation and prolong viability. They represent involvement of multiple tyrosine kinase oncogenes in excellent potential drug targets, and it is likely that acute and chronic leukemias, lymphomas, and myelo- additional mutations will be identi®ed in other kinases, mas. In each case, the tyrosine kinase activity of the their immediate downstream targets, or in proteins oncogene is constitutively activated by mutations that regulating their function. -

Anti-GRAP Antibody (ARG63124)
Product datasheet [email protected] ARG63124 Package: 100 μg anti-GRAP antibody Store at: -20°C Summary Product Description Goat Polyclonal antibody recognizes GRAP Tested Reactivity Hu Predict Reactivity Ms, Rat, Cow Tested Application WB Host Goat Clonality Polyclonal Isotype IgG Target Name GRAP Antigen Species Human Immunogen C-GFFPRSYVQPVHL Conjugation Un-conjugated Alternate Names GRB2-related adapter protein Application Instructions Application table Application Dilution WB 0.3 - 1 µg/ml Application Note WB: Recommend incubate at RT for 1h. * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. Calculated Mw 25 kDa Properties Form Liquid Purification Purified from goat serum by ammonium sulphate precipitation followed by antigen affinity chromatography using the immunizing peptide. Buffer Tris saline (pH 7.3), 0.02% Sodium azide and 0.5% BSA Preservative 0.02% Sodium azide Stabilizer 0.5% BSA Concentration 0.5 mg/ml Storage instruction For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C or below. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. www.arigobio.com 1/2 Note For laboratory research only, not for drug, diagnostic or other use. Bioinformation Database links GeneID: 10750 Human Swiss-port # Q13588 Human Background This gene encodes a member of the GRB2/Sem5/Drk family and functions as a cytoplasmic signaling protein which contains an SH2 domain flanked by two SH3 domains. -
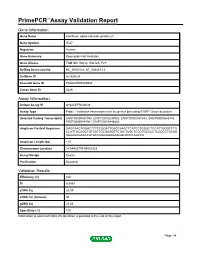
Primepcr™Assay Validation Report
PrimePCR™Assay Validation Report Gene Information Gene Name interferon, alpha-inducible protein 27 Gene Symbol IFI27 Organism Human Gene Summary Description Not Available Gene Aliases FAM14D, ISG12, ISG12A, P27 RefSeq Accession No. NC_000014.8, NT_026437.12 UniGene ID Hs.532634 Ensembl Gene ID ENSG00000165949 Entrez Gene ID 3429 Assay Information Unique Assay ID qHsaCEP0024638 Assay Type Probe - Validation information is for the primer pair using SYBR® Green detection Detected Coding Transcript(s) ENST00000557098, ENST00000298902, ENST00000557634, ENST00000555744, ENST00000444961, ENST00000448882 Amplicon Context Sequence GAGCAACTGGACTCTCCGGATTGACCAAGTTCATCCTGGGCTCCATTGGGTCTG CCATTGCGGCTGTCATTGCGAGGTTCTACTAGCTCCCTGCCCCTCGCCCTGCAG AGAAGAGAACCATGCCAGGGGAGAAGGCACCCAGCCA Amplicon Length (bp) 115 Chromosome Location 14:94582779-94582923 Assay Design Exonic Purification Desalted Validation Results Efficiency (%) 100 R2 0.9997 cDNA Cq 23.09 cDNA Tm (Celsius) 86 gDNA Cq 23.06 Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 1/4 PrimePCR™Assay Validation Report IFI27, Human Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak Melt curve analysis of above amplification Standard Curve Standard curve generated using 20 million copies of template diluted 10-fold to 20 copies Page 2/4 PrimePCR™Assay Validation Report Products used to generate validation data Real-Time PCR Instrument CFX384 Real-Time PCR Detection System Reverse Transcription Reagent iScript™ Advanced cDNA Synthesis Kit for RT-qPCR Real-Time PCR Supermix SsoAdvanced™ SYBR® Green Supermix Experimental Sample qPCR Human Reference Total RNA Data Interpretation Unique Assay ID This is a unique identifier that can be used to identify the assay in the literature and online. Detected Coding Transcript(s) This is a list of the Ensembl transcript ID(s) that this assay will detect. -

Biological Databases What Is a Database ?
BIOLOGICAL DATABASES WHAT IS A DATABASE ? A structured collection of data held in computer storage; esp. one that incorporates software to make it accessible in a variety of ways; any large collection of information. A collection of data structured searchable (index) -> table of contents updated periodically (release) -> new edition cross-referenced (hyperlinks ) -> links with other db Includes also associated tools (software) necessary for access, updating, information insertion, information deletion…. A database consists of basic units called records or entries. Each record consists of fields, which hold pre-defined data related to the record. For example, a protein database would have protein sequences as records and protein properties as fields (e.g., name of protein, length, amino-acid sequence, …) 2 DATABASES ON THE INTERNET Biological databases often have web interfaces, which allow users to send queries to the databases. Some databases can be accessed by different web servers, each offering a different interface. request query web page result User Web server Database server 3 WHY BIOLOGICAL DATABASES ? Exponential growth in biological data. Data (genomic sequences, 3D structures, 2D gel analysis, MS analysis, Microarrays….) are no longer published in a conventional manner, but directly submitted to databases. Essential tools for biological research. The only way to publish massive amounts of data without using all the paper in the world. 4 NUCLEOTIDES 5 COMPLETE GENOMES Until 2018: Eukaryotes 5262 Prokaryotes 131446 Viruses 14027 6 SOME STATISTICS More than 1000 different ‘biological’ databases Variable size: <100Kb to >20Gb DNA: > 20 Gb Protein: 1 Gb 3D structure: 5 Gb Other: smaller Update frequency: daily to annually to seldom to forget about it . -

Genetic Drivers of Pancreatic Islet Function
| INVESTIGATION Genetic Drivers of Pancreatic Islet Function Mark P. Keller,*,1 Daniel M. Gatti,†,1 Kathryn L. Schueler,* Mary E. Rabaglia,* Donnie S. Stapleton,* Petr Simecek,† Matthew Vincent,† Sadie Allen,‡ Aimee Teo Broman,§ Rhonda Bacher,§ Christina Kendziorski,§ Karl W. Broman,§ Brian S. Yandell,** Gary A. Churchill,†,2 and Alan D. Attie*,2 *Department of Biochemistry, §Department of Biostatistics and Medical Informatics, and **Department of Horticulture, University of Wisconsin–Madison, Wisconsin 53706-1544, †The Jackson Laboratory, Bar Harbor, Maine 06409, and ‡Maine School of Science and Mathematics, Limestone, Maine 06409, ORCID IDs: 0000-0002-7405-5552 (M.P.K.); 0000-0002-4914-6671 (K.W.B.); 0000-0001-9190-9284 (G.A.C.); 0000-0002-0568-2261 (A.D.A.) ABSTRACT The majority of gene loci that have been associated with type 2 diabetes play a role in pancreatic islet function. To evaluate the role of islet gene expression in the etiology of diabetes, we sensitized a genetically diverse mouse population with a Western diet high in fat (45% kcal) and sucrose (34%) and carried out genome-wide association mapping of diabetes-related phenotypes. We quantified mRNA abundance in the islets and identified 18,820 expression QTL. We applied mediation analysis to identify candidate causal driver genes at loci that affect the abundance of numerous transcripts. These include two genes previously associated with monogenic diabetes (PDX1 and HNF4A), as well as three genes with nominal association with diabetes-related traits in humans (FAM83E, IL6ST, and SAT2). We grouped transcripts into gene modules and mapped regulatory loci for modules enriched with transcripts specific for a-cells, and another specific for d-cells. -

IFI27 May Predict and Evaluate the Severity of Respiratory Syncytial Virus
Gao et al. Hereditas (2021) 158:3 https://doi.org/10.1186/s41065-020-00167-5 RESEARCH Open Access IFI27 may predict and evaluate the severity of respiratory syncytial virus infection in preterm infants Junyan Gao1, Xueping Zhu2, Mingfu Wu1, Lijun Jiang1, Fudong Wang1 and Shan He2,3* Abstract Background: Preterm infants are a special population that vulnerable to respiratory syncytial virus (RSV) infection and the lower respiratory tract infections (LRTIs) caused by RSV could be severe and even life-threating. The purpose of the present study was to identify candidate genes of preterm infants who are susceptible to RSV infection and provide a new insight into the pathogenesis of RSV infection. Methods: Three datasets (GSE77087, GSE69606 and GSE41374) containing 183 blood samples of RSV infected patients and 33 blood samples of healthy controls from Gene Expression Omnibus (GEO) database were downloaded and the differentially expressed genes (DEGs) were screened out. The function and pathway enrichments were analyzed through Database for Annotation, Visualization and Integrated Discovery (DAVID) website. The protein-protein interaction (PPI) network for DEGs was constructed through Search Tool for the Retrieval of Interacting Genes (STRING). The module analysis was performed by Cytoscape software and hub genes were identified. Clinical verification was employed to verify the expression level of top five hub genes among 72 infants including 50 RSV infected patients and 22 non-RSV-infected patients hospitalized in our center. Further, the RSV infected infants with high-expression IFI27 and those with low-expression IFI27 were compared (defined as higher or lower than the median mRNA level). -

Analysis of the Indacaterol-Regulated Transcriptome in Human Airway
Supplemental material to this article can be found at: http://jpet.aspetjournals.org/content/suppl/2018/04/13/jpet.118.249292.DC1 1521-0103/366/1/220–236$35.00 https://doi.org/10.1124/jpet.118.249292 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS J Pharmacol Exp Ther 366:220–236, July 2018 Copyright ª 2018 by The American Society for Pharmacology and Experimental Therapeutics Analysis of the Indacaterol-Regulated Transcriptome in Human Airway Epithelial Cells Implicates Gene Expression Changes in the s Adverse and Therapeutic Effects of b2-Adrenoceptor Agonists Dong Yan, Omar Hamed, Taruna Joshi,1 Mahmoud M. Mostafa, Kyla C. Jamieson, Radhika Joshi, Robert Newton, and Mark A. Giembycz Departments of Physiology and Pharmacology (D.Y., O.H., T.J., K.C.J., R.J., M.A.G.) and Cell Biology and Anatomy (M.M.M., R.N.), Snyder Institute for Chronic Diseases, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada Received March 22, 2018; accepted April 11, 2018 Downloaded from ABSTRACT The contribution of gene expression changes to the adverse and activity, and positive regulation of neutrophil chemotaxis. The therapeutic effects of b2-adrenoceptor agonists in asthma was general enriched GO term extracellular space was also associ- investigated using human airway epithelial cells as a therapeu- ated with indacaterol-induced genes, and many of those, in- tically relevant target. Operational model-fitting established that cluding CRISPLD2, DMBT1, GAS1, and SOCS3, have putative jpet.aspetjournals.org the long-acting b2-adrenoceptor agonists (LABA) indacaterol, anti-inflammatory, antibacterial, and/or antiviral activity. Numer- salmeterol, formoterol, and picumeterol were full agonists on ous indacaterol-regulated genes were also induced or repressed BEAS-2B cells transfected with a cAMP-response element in BEAS-2B cells and human primary bronchial epithelial cells by reporter but differed in efficacy (indacaterol $ formoterol . -

IFI27 May Predict and Evaluate the Severity of Respiratory Syncytial Virus Infection in Preterm Infants
IFI27 May Predict and Evaluate the Severity of Respiratory Syncytial Virus Infection in Preterm Infants Junyan Gao Aliated Hospital of Yangzhou University https://orcid.org/0000-0001-6678-6147 Xueping Zhu Children's Hospital of Soochow University Mingfu Wu Aliated Hospital of Yangzhou University Lijun Jiang Aliated Hospital of Yangzhou University Fudong Wang Aliated Hospital of Yangzhou University Shan He ( [email protected] ) Research Keywords: respiratory syncytial virus, preterm infants, differentially expressed genes, protein‐protein interaction, pathway enrichment, interferon alpha inducible protein 27 Posted Date: July 15th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-42880/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published on January 2nd, 2021. See the published version at https://doi.org/10.1186/s41065-020-00167-5. Page 1/21 Abstract Background: Preterm infants are a special population that vulnerable to respiratory syncytial virus (RSV) infection and the lower respiratory tract infection (LRTIs) caused by RSV could be severe and even life- threating. The purpose of the present study was to identify candidate genes of preterm infants who are susceptible to RSV infection and provide a new insight into the pathogenesis of RSV infection. Methods: Three datasets (GSE77087, GSE69606 and GSE41374) containing 183 blood samples of RSV infected patients and 33 blood samples of healthy controls from Gene Expression Omnibus (GEO) database were downloaded and the differentially expressed genes (DEGs) were screened out. The function and pathway enrichments were analyzed through Database for Annotation, Visualization and Integrated Discovery (DAVID) website.