Open Full Page
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Temperature-Dependent Effectiveness of Platinum-Based
cells Article The Temperature-Dependent Effectiveness of Platinum-Based Drugs Mitomycin-C and 5-FU during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Colorectal Cancer Cell Lines Roxan F.C.P.A. Helderman 1,2 , Daan R. Löke 2, Jan Verhoeff 3 , Hans M. Rodermond 1, Gregor G.W. van Bochove 1, Menno Boon 1, Sanne van Kesteren 1, Juan J. Garcia Vallejo 3, H. Petra Kok 2, Pieter J. Tanis 4 , Nicolaas A.P. Franken 1,2 , Johannes Crezee 2 and Arlene L. Oei 1,2,* 1 Laboratory for Experimental Oncology and Radiobiology (LEXOR), Center for Experimental and Molecular Medicine (CEMM), Amsterdam University Medical Centers (UMC), University of Amsterdam, Cancer Center Amsterdam, P.O. Box 22700, 1100 DE Amsterdam, The Netherlands; [email protected] (R.F.C.P.A.H.); [email protected] (H.M.R.); [email protected] (G.G.W.v.B.); [email protected] (M.B.); [email protected] (S.v.K.); [email protected] (N.A.P.F.) 2 Department of Radiation Oncology, Amsterdam UMC, University of Amsterdam, P.O. Box 22700, 1100 DE Amsterdam, The Netherlands; [email protected] (D.R.L.); [email protected] (H.P.K.); [email protected] (J.C.) 3 Department of Molecular Cell Biology & Immunology, Amsterdam Infection & Immunity Institute and Cancer Center Amsterdam, Amsterdam UMC, P.O. Box 7057, 1007 MB Amsterdam, The Netherlands; j.verhoeff@amsterdamumc.nl (J.V.); [email protected] (J.J.G.V.) 4 Department for Surgery, Amsterdam UMC, University of Amsterdam, Cancer Center Amsterdam, P.O. -

Arsenic Trioxide Is Highly Cytotoxic to Small Cell Lung Carcinoma Cells
160 Arsenic trioxide is highly cytotoxic to small cell lung carcinoma cells 1 1 Helen M. Pettersson, Alexander Pietras, effect of As2O3 on SCLC growth, as suggested by an Matilda Munksgaard Persson,1 Jenny Karlsson,1 increase in neuroendocrine markers in cultured cells. [Mol Leif Johansson,2 Maria C. Shoshan,3 Cancer Ther 2009;8(1):160–70] and Sven Pa˚hlman1 1Center for Molecular Pathology, CREATE Health and 2Division of Introduction Pathology, Department of Laboratory Medicine, Lund University, 3 Lung cancer is the most frequent cause of cancer deaths University Hospital MAS, Malmo¨, Sweden; and Department of f Oncology-Pathology, Cancer Center Karolinska, Karolinska worldwide and results in 1 million deaths each year (1). Institute and Hospital, Stockholm, Sweden Despite novel treatment strategies, the 5-year survival rate of lung cancer patients is only f15%. Small cell lung carcinoma (SCLC) accounts for 15% to 20% of all lung Abstract cancers diagnosed and is a very aggressive malignancy Small cell lung carcinoma (SCLC) is an extremely with early metastatic spread (2). Despite an initially high aggressive form of cancer and current treatment protocols rate of response to chemotherapy, which currently com- are insufficient. SCLC have neuroendocrine characteristics bines a platinum-based drug with another cytotoxic drug and show phenotypical similarities to the childhood tumor (3, 4), relapses occur in the absolute majority of SCLC neuroblastoma. As multidrug-resistant neuroblastoma patients. At relapse, the efficacy of further chemotherapy is cells are highly sensitive to arsenic trioxide (As2O3) poor and the need for alternative treatments is obvious. in vitro and in vivo, we here studied the cytotoxic effects Arsenic-containing compounds have been used in tradi- of As2O3 on SCLC cells. -
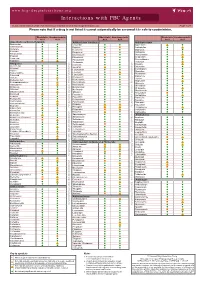
Interactions with PBC Agents
www.hep-druginteractions.org Interactions with PBC Agents Charts created March 2020. Full information available at www.hep-druginteractions.org Page 1 of 4 Please note that if a drug is not listed it cannot automatically be assumed it is safe to coadminister. Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Acid Acid Acid Acid Acid Acid Anaesthetics & Muscle Relaxants Antibacterials (continued) Antidepressants Bupivacaine Cloxacillin Agomelatine Cisatracurium Dapsone Amitriptyline Isoflurane Delamanid Bupropion Ketamine Ertapenem Citalopram Nitrous oxide Erythromycin Clomipramine Propofol Ethambutol Desipramine Thiopental Flucloxacillin Desvenlafaxine Tizanidine Gentamicin Dosulepin Analgesics Imipenem Doxepin Aceclofenac Isoniazid Duloxetine Alfentanil Escitalopram Aspirin Levofloxacin Linezolid Fluoxetine Buprenorphine Fluvoxamine Lymecycline distribution. Celecoxib Imipramine Meropenem Codeine Lithium Methenamine Dexketoprofen Maprotiline Metronidazole Dextropropoxyphene Mianserin Moxifloxacin Diamorphine Milnacipran Diclofenac Nitrofurantoin only. Not for distribution. for only. Not Mirtazapine Diflunisal Norfloxacin Moclobemide Dihydrocodeine Ofloxacin Nefazodone Etoricoxib Penicillin V Nortriptyline Fentanyl Piperacillin Paroxetine Flurbiprofen Pivmecillinam Sertraline Hydrocodone use ersonal Pyrazinamide Tianeptine Hydromorphone Rifabutin Trazodone Ibuprofen Rifampicin -

CARBOPLATIN- Carboplatin Injection Accord Healthcare, Inc
CARBOPLATIN- carboplatin injection Accord Healthcare, Inc. --------- CARBOplatin Injection Rxo nly Carboplatin injection should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Appropriate management of therapy and complications is possible only when adequate treatment facilities are readily available. Bone marrow suppression is dose related and may be severe, resulting in infection and/or bleeding. Anemia may be cumulative and may require transfusion support. Vomiting is another frequent drug related side effect. Anaphylactic-like reactions to carboplatin have been reported and may occur within minutes of carboplatin injection administration. Epinephrine, corticosteroids, and antihistamines have been employed to alleviate symptoms. DESCRIPTION Carboplatin injection is supplied as a sterile, pyrogen-free, 10 mg/mL aqueous solution of carboplatin, USP. Carboplatin, USP is a platinum coordination compound. The chemical name for carboplatin, USP is platinum, diammine [1,1-cyclobutanedicarboxylato(2-)-0,0']-,(SP-4-2), and carboplatin, USP has the following structural formula: C6H12N2O4Pt M.W. 371.25 Carboplatin, USP is a crystalline powder. It is soluble in water at a rate of approximately 14 mg/mL, and the pH of a 1% solution is 5 to 7. It is virtually insoluble in ethanol, acetone, and dimethylacetamide. CLINICAL PHARMACOLOGY Carboplatin, like cisplatin, produces predominantly interstrand DNA cross-links rather than DNA-protein cross-links. This effect is apparently cell-cycle nonspecific. The aquation of 2 carboplatin, which is thought to produce the active species, occurs at a slower rate than in the case of cisplatin. Despite this difference, it appears that both carboplatin and cisplatin induce equal numbers of drug-DNA cross-links, causing equivalent lesions and biological effects. -

Drug-Induced Sexual Dysfunction in Men and Women
VOLUME 36 : NUMBER 2 : APRIL 2013 ARTICLE Drug-induced sexual dysfunction in men and women Helen M Conaglen whether the clinician is willing to ask about sexual Clinical psychologist and SUMMARY issues and does so in a sensitive way.7,8 Senior research fellow Many medical conditions and their treatments Patients on long-term medications may not be John V Conaglen aware that their sexual problems have developed Endocrinologist and contribute to sexual dysfunction. as a result of their treatment. Conversely some may Associate professor in Commonly implicated drugs include Medicine blame their drugs for sexual problems which are due Sexual Health Research Unit antihypertensives, antidepressants, to relationship difficulties or other stressors. Some Waikato Clinical School antipsychotics and antiandrogens. doctors consider that asking patients if they had Faculty of Medical and Understanding the potential for drug-induced noticed any sexual adverse effects from their drugs Health Sciences University of Auckland sexual problems and their negative impact may ‘suggest’ them to the patient, and possibly result on adherence to treatment will enable the in non-adherence. Patients attributing their sexual clinician to tailor treatments for the patient problems to their drugs are less likely to continue the Key words treatment even when necessary for their health.9 The antidepressants, and his or her partner. consultation should include discussion of the patient’s antihypertensives, Encouraging a discussion with the patient sexual issues so these can be considered in treatment antipsychotics, arousal, about sexual function and providing erectile dysfunction, decisions. hypoactive sexual desire strategies to manage the problem are critical disorder, male impotence, to good clinical care. -

BC Cancer Protocol Summary for Neoadjuvant Or Adjuvant Therapy for Breast Cancer Using Docetaxel, Carboplatin, and Trastuzumab
BC Cancer Protocol Summary for Neoadjuvant or Adjuvant Therapy for Breast Cancer Using DOCEtaxel, CARBOplatin, and Trastuzumab Protocol Code BRAJDCARBT Tumour Group Breast Contact Physician Dr. Susan Ellard ELIGIBILITY: . ECOG 0-1 . Node positive or high risk node negative, including patient with T1b disease (T1a still requires CAP approval) . HER-2 over-expression defined as either IHC3+, or FISH amplification ratio greater than or equal to 2 per BC Cancer central laboratory . Adequate renal and hepatic function . Adequate hematological parameters (ANC greater than 1.5 x 109/L and platelets greater than 100 x 109/L) . No signs or symptoms of cardiac disease. LVEF greater than or equal to 50%* * If the LVEF is between 45-50%, the oncologist may decide to treat based on clinical assessment EXCLUSIONS: . ECOG 2-4 . Stage IV disease (please refer to advanced regimens) . Significant hepatic dysfunction, contraindicating DOCEtaxel . Significant cardiovascular disease and/or LVEF less than 50%; if initial reading is less than 50%, physician may consider repeating for validity, or assessing LVEF by the other modality, e.g. echocardiogram instead of MUGA . greater than or equal to grade 2 sensory or motor neuropathy . Pregnancy or lactation TESTS: . Baseline: CBC & diff, platelets, bilirubin, GGT, ALT, LDH, alkaline phosphatase, creatinine, (see Precaution #5 for guidelines regarding hepatic dysfunction and DOCEtaxel), suggested: nuclear renogram for GFR (if available locally, and not previously done) . Before each treatment cycle: CBC & diff, platelets, creatinine . MUGA scan or echocardiogram: prior to first treatment with trastuzumab and every 3-4 months until completion of treatment per the discretion of the treating physician. -

(12) United States Patent (10) Patent No.: US 9.498,431 B2 Xu Et Al
USOO9498431B2 (12) United States Patent (10) Patent No.: US 9.498,431 B2 Xu et al. (45) Date of Patent: Nov. 22, 2016 (54) CONTROLLED RELEASING COMPOSITION 7,053,134 B2 * 5/2006 Baldwin et al. .............. 522,154 2004/0058056 A1 3/2004 Osaki et al. ................... 427.2.1 (76) Inventors: Jianjian Xu, Hefei (CN); Shiliang 2005/0037047 A1 2/2005 Song Wang, Hefei (CN); Manzhi Ding 2007/0055364 A1* 3/2007 Hossainy .................. A61F 2/82 s: s s 623, 1.38 Hefei (CN) 2008/0274194 A1* 11/2008 Miller .................... A61K 9.146 424/489 (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 FOREIGN PATENT DOCUMENTS U.S.C. 154(b) by 0 days. CN 1208.610 A 2, 1999 (21) Appl. No.: 13/133,656 EP O251680 A2 1, 1988 JP S63-22516. A 1, 1988 JP H1O-310518 A 11, 1998 (22) PCT Filed: Dec. 10, 2009 WO 96,10395 A1 4f1996 WO WO 2005.000277 A1 * 1, 2005 (86). PCT No.: PCT/CN2009/075468 WO 2007 115045 A2 10, 2007 WO 2008/OO2657 A2 1, 2008 S 371 (c)(1), WO 2008OO2657 A2 1, 2008 (2), (4) Date: Jun. 9, 2011 WO 2008041246 A2 4/2008 (87) PCT Pub. No.: WO2010/066203 OTHER PUBLICATIONS PCT Pub. Date: Jun. 17, 2010 Crowley and Zhang, Pharmaceutical Application of Hot Melt Extru (65) Prior Publication Data sion: Part I, Drug Development and Industrial Pharmacy, 2007. 33:909-926.* US 2011/024.4043 A1 Oct. 6, 2011 The Use of Poly (L-Lactide) and RGD Modified Microspheres as Cell Carriers in a Flow Intermittency Bioreactor for Tissue Engi (30) Foreign Application Priority Data neering Cartilage. -

Chemotherapy Protocol LUNG CANCER – SMALL CELL (SCLC) CARBOPLATIN (AUC6)-ETOPOSIDE (Intravenous / Oral) Regimen SCLC – Carbo
Chemotherapy Protocol LUNG CANCER – SMALL CELL (SCLC) CARBOPLATIN (AUC6)-ETOPOSIDE (Intravenous / Oral) Regimen SCLC – Carboplatin (AUC6)-Etoposide IV/PO Indication First line treatment of SCLC WHO Performance status 0, 1, 2, 3 Toxicity Drug Adverse Effect Carboplatin Neuropathy, hypersensitivity Etoposide Hypotension on rapid infusion, hyperbilirubinaemia The adverse effects listed are not exhaustive. Please refer to the relevant Summary of Product Characteristics for full details. Monitoring Disease A baseline chest x-ray should be performed before starting treatment and up to date (ideally within 1 month) cross section imaging should also be performed Regimen EDTA or calculated creatinine clearance before the 1st cycle. FBC, LFTs and U&Es prior to each cycle A chest x-ray should be performed before each cycle Dose Modifications The dose modifications listed are for haematological, liver and renal function only. Dose adjustments may be necessary for other toxicities as well. In principle all dose reductions due to adverse drug reactions should not be re- escalated in subsequent cycles without consultant approval. It is also a general rule Version 1.3 (December 2013) Page 1 of 7 SCLC- Carboplatin (AUC6)-Etoposide IV/PO for chemotherapy that if a third dose reduction is necessary treatment should be stopped. Please discuss all dose reductions / delays with the relevant consultant before prescribing, if appropriate. The approach may be different depending on the clinical circumstances. The following is a general guide only. Haematology Prior to prescribing on day one of cycle one the following criteria must be met; Criteria Eligible Level Neutrophil equal to or more than 1.5x109/L Platelets equal to or more than 100x109/L Consider blood transfusion if patient symptomatic of anaemia or haemoglobin of less than 8g/dL Subsequently if the neutrophils are less than 1x109/L then in the first instance delay treatment for seven days. -

Dative Metabolism of Cortisol in Humans Kunihi
臨 床 薬 理JPn J Clin Pharmacol Ther 18 (3) Sept 1987 509 Comparison of the Inhibitory Effects of Famotidine and Cimetidine on Hepatic Oxi- dative Metabolism of Cortisol in Humans Kunihiko MORITA* Hiroki KONISHI* Takeshi ONO* and Harumi SHIMAKAWA* (Receivedon Dec.19, 1986) * Hospital Pharmacy, Shiga Universityof Medical Science, Tsukinowa-cho,Seta, Ohtsu520-21, Japan The inhibitory effect of famotidine, a new H2-receptor antagonist, on hepatic oxidative metabolism of cortisol in six healthy volunteers was compared with that of cimetidine by monitoring the change in urinary 6ƒÀ-hydroxycortisol (6ƒÀ-OHF), an oxidative metabolite of cortisol. The ratio of 6ƒÀ-OHF to 17-hydroxycorticosteroids (17-OHCS) in urine was measured before, during, and after treatment with famotidine and cimetidine for 3 days in a cross-over study. The ratio was decreased by 25% -35% of the original level after 1-3 days of oral treatment with cimetidine (800 mg, b. i. d.). The reduction vanished within 2 days after the last dose of cimetidine. The ratio was not significantly changed during oral treatment with famotidine (40 mg, b. i. d.). These findings indicate that famotidine, in contrast to cimetidine, does not affect the hepatic oxidative metabolism of cortisol in man, and it is suggested that famotidine does not affect the hepatic drug-metabolizing capacity in humans. Key words: famotidine, cimetidine, H2-receptor antangonist, 6ƒÀ-hydroxycortisol, enzyme inhibition been shown to inhibit the hepatic elimination of a Introduction number of drugs coadministred.1-4) A number of Cimetidine, a histamine H2-receptor antagonist studies have demonstrated that this inhibitory widely used for the treatment of peptic ulcer, has action of cimetidine is based on its high affinity for cytochrome P-450 in hepatic microsomes *滋 賀医科大学医学部附属病院薬剤部 arising from imidazole and cyano groups.5-7) 〓520-21滋 賀県大津市瀬田月輪町 Ranitidine, an H2-receptor antagonist developed 510 hospital pharmacists, aged 24 to 35 years old (mean 28), participated in the study. -

Stability of Carboplatin and Oxaliplatin in Their Infusion Solutions Is Due to Self-Association
Syracuse University SURFACE Chemistry - Faculty Scholarship College of Arts and Sciences 2011 Stability of Carboplatin and Oxaliplatin in their Infusion Solutions is Due to Self-Association Anthony J. Di Pasqua Syracuse University Deborah J. Kerwood Syracuse University Yi Shi Syracuse University Jerry Goodisman Syracuse University James C. Dabrowiak Syracuse University Follow this and additional works at: https://surface.syr.edu/che Part of the Chemistry Commons Recommended Citation Di Pasqua, Anthony J.; Kerwood, Deborah J.; Shi, Yi; Goodisman, Jerry; and Dabrowiak, James C., "Stability of Carboplatin and Oxaliplatin in their Infusion Solutions is Due to Self-Association" (2011). Chemistry - Faculty Scholarship. 29. https://surface.syr.edu/che/29 This Article is brought to you for free and open access by the College of Arts and Sciences at SURFACE. It has been accepted for inclusion in Chemistry - Faculty Scholarship by an authorized administrator of SURFACE. For more information, please contact [email protected]. View Article Online / Journal Homepage / Table of Contents for this issue Dalton Dynamic Article Links Transactions Cite this: Dalton Trans., 2011, 40, 4821 www.rsc.org/dalton COMMUNICATION Stability of carboplatin and oxaliplatin in their infusion solutions is due to self-association Anthony J. Di Pasqua,† Deborah J. Kerwood, Yi Shi, Jerry Goodisman and James C. Dabrowiak* Received 13th December 2010, Accepted 23rd March 2011 DOI: 10.1039/c0dt01758b Carboplatin and oxaliplatin are commonly used platinum anticancer agents that are sold as ready-to-use aqueous infusion solutions with shelf lives of 2 and 3 years, respectively. The observed rate constants for the hydrolysis of these drugs, however, are too large to account for their long shelf lives. -

Auspar Attachment 1. Product Information for Mifepristone
Attachment 1: Product information for AusPAR MS-2 Step mifepristone/misoprostol MS Health Pty Ltd PM-2013-01037-1-5 Final 13 October 2014. This Product Information was approved at the time this AusPAR was published. PRODUCT INFORMATION - MS-2 StepÔ It is very important that all patients receiving these medications are followed up by a medical practitioner, preferably the prescriber, to ensure that the medication has been effective. Even if no adverse events have occurred all patients must receive follow-up 14 to 21 days after taking mifepristone. Read the SPECIAL WARNINGS AND PRECAUTIONS FOR USE carefully. This document refers to the use of MS-2 StepÔ, which consists of Mifepristone Linepharma 200 mg tablet and GyMiso® misoprostol 200 microgram tablets in combination. These medicines are indicated in females of childbearing age for the medical termination of a developing intrauterine pregnancy, up to 63 days of gestation. The Mifepristone Linepharma 200 mg tablet component of this therapy is also used to treat another condition. For information about the treatment of the other condition, refer to the full Product Information for Mifepristone Linepharma 200 mg tablet individual product (AUST R 175671). NAME OF THE MEDICINE MS-2 StepÔ is a composite pack containing Mifepristone Linepharma 200 mg tablet and GyMiso® misoprostol 200 microgram tablets. Mifepristone Australian Approved Name (AAN): Mifepristone Chemical Structure: CH3 N CH 3 OH CH H CH3 3 H H O Molecular formula: C29H35NO2 Molecular weight: 429.6 Version:22May2014 [63days] Page 1 of 23 Attachment 1: Product information for AusPAR MS-2 Step mifepristone/misoprostol MS Health Pty Ltd PM-2013-01037-1-5 Final 13 October 2014. -

Preferred Drug List 4-Tier
Preferred Drug List 4-Tier 21NVHPN13628 Four-Tier Base Drug Benefit Guide Introduction As a member of a health plan that includes outpatient prescription drug coverage, you have access to a wide range of effective and affordable medications. The health plan utilizes a Preferred Drug List (PDL) (also known as a drug formulary) as a tool to guide providers to prescribe clinically sound yet cost-effective drugs. This list was established to give you access to the prescription drugs you need at a reasonable cost. Your out- of-pocket prescription cost is lower when you use preferred medications. Please refer to your Prescription Drug Benefit Rider or Evidence of Coverage for specific pharmacy benefit information. The PDL is a list of FDA-approved generic and brand name medications recommended for use by your health plan. The list is developed and maintained by a Pharmacy and Therapeutics (P&T) Committee comprised of actively practicing primary care and specialty physicians, pharmacists and other healthcare professionals. Patient needs, scientific data, drug effectiveness, availability of drug alternatives currently on the PDL and cost are all considerations in selecting "preferred" medications. Due to the number of drugs on the market and the continuous introduction of new drugs, the PDL is a dynamic and routinely updated document screened regularly to ensure that it remains a clinically sound tool for our providers. Reading the Drug Benefit Guide Benefits for Covered Drugs obtained at a Designated Plan Pharmacy are payable according to the applicable benefit tiers described below, subject to your obtaining any required Prior Authorization or meeting any applicable Step Therapy requirement.