New Aspects of Graft-Induced Central Plasticity and Neuroregeneration At
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NS201C Anatomy 1: Sensory and Motor Systems
NS201C Anatomy 1: Sensory and Motor Systems 25th January 2017 Peter Ohara Department of Anatomy [email protected] The Subdivisions and Components of the Central Nervous System Axes and Anatomical Planes of Sections of the Human and Rat Brain Development of the neural tube 1 Dorsal and ventral cell groups Dermatomes and myotomes Neural crest derivatives: 1 Neural crest derivatives: 2 Development of the neural tube 2 Timing of development of the neural tube and its derivatives Timing of development of the neural tube and its derivatives Gestational Crown-rump Structure(s) age (Weeks) length (mm) 3 3 cerebral vesicles 4 4 Optic cup, otic placode (future internal ear) 5 6 cerebral vesicles, cranial nerve nuclei 6 12 Cranial and cervical flexures, rhombic lips (future cerebellum) 7 17 Thalamus, hypothalamus, internal capsule, basal ganglia Hippocampus, fornix, olfactory bulb, longitudinal fissure that 8 30 separates the hemispheres 10 53 First callosal fibers cross the midline, early cerebellum 12 80 Major expansion of the cerebral cortex 16 134 Olfactory connections established 20 185 Gyral and sulcul patterns of the cerebral cortex established Clinical case A 68 year old woman with hypertension and diabetes develops abrupt onset numbness and tingling on the right half of the face and head and the entire right hemitrunk, right arm and right leg. She does not experience any weakness or incoordination. Physical Examination: Vitals: T 37.0° C; BP 168/87; P 86; RR 16 Cardiovascular, pulmonary, and abdominal exam are within normal limits. Neurological Examination: Mental Status: Alert and oriented x 3, 3/3 recall in 3 minutes, language fluent. -

Glial Cell Development and Function in Zebrafish
Downloaded from http://cshperspectives.cshlp.org/ on September 26, 2021 - Published by Cold Spring Harbor Laboratory Press Glial Cell Development and Function in Zebrafish David A. Lyons1 and William S. Talbot2 1Centre for Neuroregeneration, University of Edinburgh, Edinburgh EH16 4SB, United Kingdom 2Department of Developmental Biology, Stanford University, Stanford, California 94305 Correspondence: [email protected] The zebrafish is a premier vertebrate model system that offers many experimental advantages for in vivo imaging and genetic studies. This review provides an overview of glial cell types in the central and peripheral nervous system of zebrafish. We highlight some recent work that exploited the strengths of the zebrafish system to increase the understanding of the role of Gpr126 in Schwann cell myelination and illuminate the mechanisms controlling oligoden- drocyte development and myelination. We also summarize similarities and differences between zebrafish radial glia and mammalian astrocytes and consider the possibility that their distinct characteristics may represent extremes in a continuum of cell identity. Finally, we focus on the emergence of zebrafish as a model for elucidating the development and function of microglia. These recent studies have highlighted the power of the zebrafish system for analyzing important aspects of glial development and function. ollowing the pioneering work of George Ho and Kane 1990; Hatta et al. 1991; Grunwald FStreisinger in the early 1980s, the zebrafish and Eisen 2002), attracting many researchers -

Meninges,Cerebrospinal Fluid, and the Spinal Cord
The Nervous System SPINAL CORD Spinal Cord Continuation of CNS inferior to foramen magnum (medulla) Simpler Conducts impulses to and from brain Two way conduction pathway Reflex actions Spinal Cord Passes through vertebral canal Foramen magnum L2 Conus medullaris Filum terminale Cauda equina Cervical Cervical spinal nerves enlargement Dura and arachnoid Thoracic mater spinal nerves Lumbar enlargement Conus medullaris Lumbar Cauda spinal nerves equina Filum (a) The spinal cord and its nerve terminale Sacral roots, with the bony vertebral spinal nerves arches removed. The dura mater and arachnoid mater are cut open and reflected laterally. Figure 12.29a Spinal Cord Spinal nerves 31 pairs Cervical and lumbar enlargements The nerves serving the upper and lower limbs emerge here Cervical Cervical spinal nerves enlargement Dura and arachnoid Thoracic mater spinal nerves Lumbar enlargement Conus medullaris Lumbar Cauda spinal nerves equina Filum (a) The spinal cord and its nerve terminale Sacral roots, with the bony vertebral spinal nerves arches removed. The dura mater and arachnoid mater are cut open and reflected laterally. Figure 12.29a Spinal Cord Protection Bone, meninges, and CSF Spinal tap-inferior to second lumbar vertebra T12 Ligamentum flavum L5 Lumbar puncture needle entering subarachnoid space L4 Supra- spinous ligament L5 Filum terminale S1 Inter- Cauda equina vertebral Arachnoid Dura in subarachnoid disc matter mater space Figure 12.30 Spinal Cord Cross section Central gray matter Cortex of white matter Epidural -

Calmodulin Complexation in Neurons and Brain Degeneration in Alzheimer’S Disease
International Journal of Molecular Sciences Review The Relevance of Amyloid β-Calmodulin Complexation in Neurons and Brain Degeneration in Alzheimer’s Disease Joana Poejo 1 , Jairo Salazar 1,2, Ana M. Mata 1,3 and Carlos Gutierrez-Merino 1,3,* 1 Instituto de Biomarcadores de Patologías Moleculares, Universidad de Extremadura, 06006 Badajoz, Spain; [email protected] (J.P.); [email protected] (J.S.); [email protected] (A.M.M.) 2 Departamento de Química, Universidad Nacional Autónoma de Nicaragua-León, León 21000, Nicaragua 3 Departamento de Bioquímica y Biología Molecular y Genética, Facultad de Ciencias, Universidad de Extremadura, 06006 Badajoz, Spain * Correspondence: [email protected] Abstract: Intraneuronal amyloid β (Aβ) oligomer accumulation precedes the appearance of amyloid plaques or neurofibrillary tangles and is neurotoxic. In Alzheimer’s disease (AD)-affected brains, intraneuronal Aβ oligomers can derive from Aβ peptide production within the neuron and, also, from vicinal neurons or reactive glial cells. Calcium homeostasis dysregulation and neuronal ex- citability alterations are widely accepted to play a key role in Aβ neurotoxicity in AD. However, the identification of primary Aβ-target proteins, in which functional impairment initiating cytosolic calcium homeostasis dysregulation and the critical point of no return are still pending issues. The micromolar concentration of calmodulin (CaM) in neurons and its high affinity for neurotoxic Aβ peptides (dissociation constant ≈ 1 nM) highlight a novel function of CaM, i.e., the buffering of free Aβ concentrations in the low nanomolar range. In turn, the concentration of Aβ-CaM complexes within neurons will increase as a function of time after the induction of Aβ production, and free Aβ Citation: Poejo, J.; Salazar, J.; Mata, will rise sharply when accumulated Aβ exceeds all available CaM. -

Review of Spinal Cord Basics of Neuroanatomy Brain Meninges
Review of Spinal Cord with Basics of Neuroanatomy Brain Meninges Prof. D.H. Pauža Parts of Nervous System Review of Spinal Cord with Basics of Neuroanatomy Brain Meninges Prof. D.H. Pauža Neurons and Neuroglia Neuron Human brain contains per 1011-12 (trillions) neurons Body (soma) Perikaryon Nissl substance or Tigroid Dendrites Axon Myelin Terminals Synapses Neuronal types Unipolar, pseudounipolar, bipolar, multipolar Afferent (sensory, centripetal) Efferent (motor, centrifugal, effector) Associate (interneurons) Synapse Presynaptic membrane Postsynaptic membrane, receptors Synaptic cleft Synaptic vesicles, neuromediator Mitochondria In human brain – neurons 1011 (100 trillions) Synapses – 1015 (quadrillions) Neuromediators •Acetylcholine •Noradrenaline •Serotonin •GABA •Endorphin •Encephalin •P substance •Neuronal nitric oxide Adrenergic nerve ending. There are many 50-nm-diameter vesicles (arrow) with dark, electron-dense cores containing norepinephrine. x40,000. Cell Types of Neuroglia Astrocytes - Oligodendrocytes – Ependimocytes - Microglia Astrocytes – a part of hemoencephalic barrier Oligodendrocytes Ependimocytes and microglial cells Microglia represent the endogenous brain defense and immune system, which is responsible for CNS protection against various types of pathogenic factors. After invading the CNS, microglial precursors disseminate relatively homogeneously throughout the neural tissue and acquire a specific phenotype, which clearly distinguish them from their precursors, the blood-derived monocytes. The ´resting´ microglia -

Original Article Schistosoma Japonicum-Derived Peptide SJMHE1 Promotes Peripheral Nerve Repair Through a Macrophage-Dependent Mechanism
Am J Transl Res 2021;13(3):1290-1306 www.ajtr.org /ISSN:1943-8141/AJTR0118598 Original Article Schistosoma japonicum-derived peptide SJMHE1 promotes peripheral nerve repair through a macrophage-dependent mechanism Yongbin Ma1,2, Chuan Wei1, Xin Qi1, Yanan Pu1, Liyang Dong3, Lei Xu1, Sha Zhou1, Jifeng Zhu1, Xiaojun Chen1, Xuefeng Wang4, Chuan Su1 1State Key Lab of Reproductive Medicine, Jiangsu Key Laboratory of Pathogen Biology, Department of Pathogen Biology and Immunology, Center for Global Health, Nanjing Medical University, Nanjing 211166, Jiangsu, P. R. China; 2Department of Neurology Laboratory, Jintan Hospital, Jiangsu University, Jintan, Changzhou 213200, Jiangsu, P. R. China; 3Department of Nuclear Medicine and Institute of Oncology, The Affiliated Hospital of Jiangsu University, Zhenjiang 212000, Jiangsu, P. R. China; 4Department of Central Laboratory, The Affiliated Hospital of Jiangsu University, Zhenjiang 212000, Jiangsu, P. R. China Received July 21, 2020; Accepted December 11, 2020; Epub March 15, 2021; Published March 30, 2021 Abstract: Peripheral nerve injury, a disease that affects 1 million people worldwide every year, occurs when periph- eral nerves are destroyed by injury, systemic illness, infection, or an inherited disorder. Indeed, repair of damaged peripheral nerves is predominantly mediated by type 2 immune responses. Given that helminth parasites induce type 2 immune responses in hosts, we wondered whether helminths or helminth-derived molecules might have the potential to improve peripheral nerve repair. Here, we demonstrated that schistosome-derived SJMHE1 promoted peripheral myelin growth and functional regeneration via a macrophage-dependent mechanism and simultaneously increased the induction of M2 macrophages. Our findings highlight the therapeutic potential of schistosome-derived SJMHE1 for improving peripheral nerve repair. -

UC Riverside UC Riverside Electronic Theses and Dissertations
UC Riverside UC Riverside Electronic Theses and Dissertations Title Remote-Activated Electrical Stimulation via Piezoelectric Scaffold System for Functional Peripheral and Central Nerve Regeneration Permalink https://escholarship.org/uc/item/7hb5g2x7 Author Low, Karen Gail Publication Date 2017 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA RIVERSIDE Remote-Activated Electrical Stimulation via Piezoelectric Scaffold System for Functional Nerve Regeneration A Dissertation submitted in partial satisfaction of the requirements of for the degree of Doctor of Philosophy in Bioengineering by Karen Gail Low December 2017 Dissertation Committee: Dr. Jin Nam, Chairperson Dr. Hyle B. Park Dr. Nosang V. Myung Copyright by Karen Gail Low 2017 The Dissertation of Karen Gail Low is approved: _____________________________________________ _____________________________________________ _____________________________________________ Committee Chairperson University of California, Riverside ACKNOWLEDGEMENTS First and foremost, I would like to express my deepest appreciation to my PhD advisor and mentor, Dr. Jin Nam. I came from a background with no research experience, therefore his guidance, motivation, and ambition for me to succeed helped developed me into the researcher I am today. And most of all, I am forever grateful for his patience with all my blood, sweat and tears that went into this 5 years. He once said, “it takes pressure to make a diamond.” His words of wisdom will continue to guide me through my career. I would also like to thank my collaborator, Dr. Nosang V. Myung. He gave me the opportunity to explore a field that was completely outside of my comfort zone of biology. -

Presynaptic Gabaergic Inhibition Regulated by BDNF Contributes to Neuropathic Pain Induction
ARTICLE Received 29 Apr 2014 | Accepted 22 Sep 2014 | Published 30 Oct 2014 DOI: 10.1038/ncomms6331 OPEN Presynaptic GABAergic inhibition regulated by BDNF contributes to neuropathic pain induction Jeremy Tsung-chieh Chen1, Da Guo1, Dario Campanelli1,2, Flavia Frattini1, Florian Mayer1, Luming Zhou3, Rohini Kuner4, Paul A. Heppenstall5, Marlies Knipper2 & Jing Hu1 The gate control theory proposes the importance of both pre- and post-synaptic inhibition in processing pain signal in the spinal cord. However, although postsynaptic disinhibition caused by brain-derived neurotrophic factor (BDNF) has been proved as a crucial mechanism underlying neuropathic pain, the function of presynaptic inhibition in acute and neuropathic pain remains elusive. Here we show that a transient shift in the reversal potential (EGABA) together with a decline in the conductance of presynaptic GABAA receptor result in a reduction of presynaptic inhibition after nerve injury. BDNF mimics, whereas blockade of BDNF signalling reverses, the alteration in GABAA receptor function and the neuropathic pain syndrome. Finally, genetic disruption of presynaptic inhibition leads to spontaneous development of behavioural hypersensitivity, which cannot be further sensitized by nerve lesions or BDNF. Our results reveal a novel effect of BDNF on presynaptic GABAergic inhibition after nerve injury and may represent new strategy for treating neuropathic pain. 1 Centre for Integrative Neuroscience, Otfried-Mueller-Strasse 25, 72076 Tu¨bingen, Germany. 2 Hearing Research Centre, Elfriede Aulhornstrasse 5, 72076 Tu¨bingen, Germany. 3 Laboratory for NeuroRegeneration and Repair, Center for Neurology, Hertie Institute for Clinical Brain Research, 72076 Tu¨bingen, Germany. 4 Pharmacology Institute, University of Heidelberg, Im Neuenheimer Feld 584, 69120 Heidelberg, Germany. -

Closed-Loop Neuromodulation Restores Network Connectivity And
RESEARCH ARTICLE Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury Patrick D Ganzer1,2*, Michael J Darrow1, Eric C Meyers1,2, Bleyda R Solorzano2, Andrea D Ruiz2, Nicole M Robertson2, Katherine S Adcock3, Justin T James2, Han S Jeong2, April M Becker4, Mark P Goldberg4, David T Pruitt1,2, Seth A Hays1,2,3, Michael P Kilgard1,2,3, Robert L Rennaker II1,2,3* 1Erik Jonsson School of Engineering and Computer Science, The University of Texas at Dallas, Richardson, United States; 2Texas Biomedical Device Center, Richardson, United States; 3School of Behavioral Brain Sciences, The University of Texas at Dallas, Richardson, United States; 4Department of Neurology and Neurotherapeutics, University of Texas Southwestern Medical Center, Dallas, United States Abstract Recovery from serious neurological injury requires substantial rewiring of neural circuits. Precisely-timed electrical stimulation could be used to restore corrective feedback mechanisms and promote adaptive plasticity after neurological insult, such as spinal cord injury (SCI) or stroke. This study provides the first evidence that closed-loop vagus nerve stimulation (CLV) based on the synaptic eligibility trace leads to dramatic recovery from the most common forms of SCI. The addition of CLV to rehabilitation promoted substantially more recovery of forelimb function compared to rehabilitation alone following chronic unilateral or bilateral cervical SCI in a rat model. Triggering stimulation on the most successful movements is critical to maximize recovery. CLV enhances recovery by strengthening synaptic connectivity from remaining motor *For correspondence: networks to the grasping muscles in the forelimb. The benefits of CLV persist long after the end of [email protected] stimulation because connectivity in critical neural circuits has been restored. -
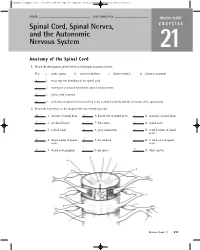
Spinal Cord, Spinal Nerves, and the Autonomic Nervous System
ighapmLre21pg211_216 5/12/04 2:24 PM Page 211 impos03 302:bjighapmL:ighapmLrevshts:layouts: NAME ___________________________________ LAB TIME/DATE _______________________ REVIEW SHEET Spinal Cord, Spinal Nerves, exercise and the Autonomic Nervous System 21 Anatomy of the Spinal Cord 1. Match the descriptions given below to the proper anatomical term: Key: a. cauda equina b. conus medullaris c. filum terminale d. foramen magnum d 1. most superior boundary of the spinal cord c 2. meningeal extension beyond the spinal cord terminus b 3. spinal cord terminus a 4. collection of spinal nerves traveling in the vertebral canal below the terminus of the spinal cord 2. Match the key letters on the diagram with the following terms. m 1. anterior (ventral) hornn 6. dorsal root of spinal nervec 11. posterior (dorsal) horn k 2. arachnoid materj 7. dura materf 12. spinal nerve a 3. central canalo 8. gray commissure i 13. ventral ramus of spinal nerve h 4. dorsal ramus of spinald 9. lateral horne 14. ventral root of spinal nerve nerve g l 5. dorsal root ganglion 10. pia materb 15. white matter o a b n c m d e l f g k h j i Review Sheet 21 211 ighapmLre21pg211_216 5/12/04 2:24 PM Page 212 impos03 302:bjighapmL:ighapmLrevshts:layouts: 3. Choose the proper answer from the following key to respond to the descriptions relating to spinal cord anatomy. Key: a. afferent b. efferent c. both afferent and efferent d. association d 1. neuron type found in posterior hornb 4. fiber type in ventral root b 2. -

Reorganization of Intact Descending Motor Circuits to Replace Lost Connections After Injury
Neurotherapeutics (2016) 13:370–381 DOI 10.1007/s13311-016-0422-x REVIEW Reorganization of Intact Descending Motor Circuits to Replace Lost Connections After Injury Kathren L. Fink1 & William B. J. Cafferty 1 Published online: 3 February 2016 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Neurons have a limited capacity to regenerate in the molecular mechanisms that drive plasticity within intact cir- adult central nervous system (CNS). The inability of damaged cuits is crucial in developing novel, potent, and specific ther- axons to re-establish original circuits results in permanent apeutics to restore function after SCI. In this review we dis- functional impairment after spinal cord injury (SCI). Despite cuss the evidence supporting a focus on exploring the capacity abortive regeneration of axotomized CNS neurons, limited of intact motor circuits to functionally repair the damaged spontaneous recovery of motor function emerges after partial CNS after SCI. SCI in humans and experimental rodent models of SCI. It is hypothesized that this spontaneous functional recovery is the result of the reorganization of descending motor pathways Keywords Spinal cord injury . Plasticity . Regeneration . spared by the injury, suggesting that plasticity of intact circuits Repair . Axon . Neuron is a potent alternative conduit to enhance functional recovery after SCI. In support of this hypothesis, several studies have shown that after unilateral corticospinal tract (CST) lesion (unilateral pyramidotomy), -

Cerebral Cortex
Cerebral Cortex • Research on the structure and function of the brain reveals that there are both specialized and diffuse areas of function • Motor and sensory areas are localized in discrete cortical areas called domains • Many higher mental functions such as memory and language appear to have overlapping domains and are more diffusely located • Broadmann areas are areas of localized function Cerebral Cortex - Generalizations • The cerebral cortex has three types of functional areas – Motor areas / control voluntary motor function – Sensory areas / provide conscious awareness of sensation – Association areas / act mainly to integrate diverse information for purposeful action • Each hemisphere is chiefly concerned with the sensory and motor functions of the opposite (contralateral) side of the body Motor Areas • Cortical areas controlling motor functions lie in the posterior part of the frontal lobes • Motor areas include the primary motor cortex, the premotor cortex, Broca’s area, and the front eye field Primary Motor Cortex • The primary motor cortex is located in the precentral gyrus of the frontal lobe of each hemisphere • Large neurons (pyramidal cells) in these gyri allow us to consciously control the precise or skill voluntary movements of our skeletal muscles Pyramidal cells • These long axons, which project to the spinal cord, form the massive Dendrites voluntary motor tracts called the pyramidal, or corticospinal tracts • All other descending motor tracts issue from brain stem nuclei and consists of chains of two, three, or