Skull-Base Foramina of the Middle Cranial Fossa: Reassessment of Normal Variation with High-Resolution CT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Middle Cranial Fossa Sphenoidal Region Dural Arteriovenous Fistulas: Anatomic and Treatment Considerations
ORIGINAL RESEARCH INTERVENTIONAL Middle Cranial Fossa Sphenoidal Region Dural Arteriovenous Fistulas: Anatomic and Treatment Considerations Z.-S. Shi, J. Ziegler, L. Feng, N.R. Gonzalez, S. Tateshima, R. Jahan, N.A. Martin, F. Vin˜uela, and G.R. Duckwiler ABSTRACT BACKGROUND AND PURPOSE: DAVFs rarely involve the sphenoid wings and middle cranial fossa. We characterize the angiographic findings, treatment, and outcome of DAVFs within the sphenoid wings. MATERIALS AND METHODS: We reviewed the clinical and radiologic data of 11 patients with DAVFs within the sphenoid wing that were treated with an endovascular or with a combined endovascular and surgical approach. RESULTS: Nine patients presented with ocular symptoms and 1 patient had a temporal parenchymal hematoma. Angiograms showed that 5 DAVFs were located on the lesser wing of sphenoid bone, whereas the other 6 were on the greater wing of the sphenoid bone. Multiple branches of the ICA and ECA supplied the lesions in 7 patients. Four patients had cortical venous reflux and 7 patients had varices. Eight patients were treated with transarterial embolization using liquid embolic agents, while 3 patients were treated with transvenous embo- lization with coils or in combination with Onyx. Surgical disconnection of the cortical veins was performed in 2 patients with incompletely occluded DAVFs. Anatomic cure was achieved in all patients. Eight patients had angiographic and clinical follow-up and none had recurrence of their lesions. CONCLUSIONS: DAVFs may occur within the dura of the sphenoid wings and may often have a presentation similar to cavernous sinus DAVFs, but because of potential associations with the cerebral venous system, may pose a risk for intracranial hemorrhage. -

MR Imaging of the Orbital Apex
J Korean Radiol Soc 2000;4 :26 9-0 6 1 6 MR Imaging of the Orbital Apex: An a to m y and Pat h o l o g y 1 Ho Kyu Lee, M.D., Chang Jin Kim, M.D.2, Hyosook Ahn, M.D.3, Ji Hoon Shin, M.D., Choong Gon Choi, M.D., Dae Chul Suh, M.D. The apex of the orbit is basically formed by the optic canal, the superior orbital fis- su r e , and their contents. Space-occupying lesions in this area can result in clinical d- eficits caused by compression of the optic nerve or extraocular muscles. Even vas c u l a r changes in the cavernous sinus can produce a direct mass effect and affect the orbit ap e x. When pathologic changes in this region is suspected, contrast-enhanced MR imaging with fat saturation is very useful. According to the anatomic regions from which the lesions arise, they can be classi- fied as belonging to one of five groups; lesions of the optic nerve-sheath complex, of the conal and intraconal spaces, of the extraconal space and bony orbit, of the cav- ernous sinus or diffuse. The characteristic MR findings of various orbital lesions will be described in this paper. Index words : Orbit, diseases Orbit, MR The apex of the orbit is a complex region which con- tains many nerves, vessels, soft tissues, and bony struc- Anatomy of the orbital apex tures such as the superior orbital fissure and the optic canal (1-3), and is likely to be involved in various dis- The orbital apex region consists of the optic nerve- eases (3). -

The Morphometric Study of Occurrence and Variations of Foramen Ovale S
Research Article The morphometric study of occurrence and variations of foramen ovale S. Ajrish George*, M. S. Thenmozhi ABSTRACT Background: Foramen vale is one of the important foramina present in the sphenoid bone. Anatomically it is located in the greater wing of the sphenoid bone. The foramen ovale is situated posterolateral to the foramen rotundum and anteromedial to the foramen spinosum. The foramen spinosum is present posterior to the foramen ovale. The carotid canal is present posterior and medial to the foramen spinosum and the foramen rotundum is present anterior to the foramen ovale. The structures which pass through the foramen ovale are the mandibular nerve, emissary vein, accessory middle meningeal artery, and lesser petrosal nerve. The sphenoid bone has a body, a pair of greater wing, pair of lesser wing, pair of lateral pterygoid plate, and a pair of medial pterygoid plate. Aim: The study involves the assessment of any additional features in foramen ovale in dry South Indian skulls. Materials and Methods: This study involves examination of dry adult skulls. First, the foramen ovale is located, and then it is carefully examined for presence of alterations and additional features, and is recorded following computing the data and analyzing it. Results: The maximum length of foramen ovale on the right and left was 10.1 mm, 4.3 mm, respectively. The minimum length of the foramen in right and left was 9.1 mm, 3.2 mm, respectively. The maximum width of foramen ovale on the right and left was 4.8 mm and 2.3 mm, respectively. The minimum width of the foramen in the right and the left side was 5.7 mm and 2.9 mm, respectively. -

The Normal Dimensions of the Sella Turcica in North Karnataka Region- a Computed Tomographic Study Lohit V Shaha*, Babasaheb G Patil**, Sanjeev I Kolagi***
Original Article Pravara Med Rev 2018;10(3) The normal dimensions of the Sella Turcica in North Karnataka region- A Computed tomographic study Lohit V Shaha*, Babasaheb G Patil**, Sanjeev I Kolagi*** Abstract Aim of the study: Sella turcica is an important structure in middle cranial fossa. It is a saddle shaped concavity in the body of sphenoid bone. It is bounded by dura of cavernous sinuses bilaterally, the lamina dura and dorsum sellae posteriorly and the tuberculum sellae and planum sphenoidale anteriorly. The present study was undertaken to study the normal dimensions of sella turcica morphometry. Material and methods: This observational study was conducted in S Nijalingappa medical college and HSK hospital, Bagalkot. 1650 computed tomographic images of healthy Indians aged 21-70 years were collected. Radiant Dicom viewer software was used to determine the linear dimensions of sella turcica. Data was analysed using t test and ANOVA with Epi Info software. Results: The mean values (in millimeter) of length, width and height of sella turcica in different age groups was 8.80 ± 1.65, 10.83 ± 1.35 and 8.52 ± 1.50. Conclusion: The dimensions of sella turcica vary in different populations and these findings could form an initial database for Indian population which may provide a good anatomical knowledge during objective evaluation and detection of pathological conditions of sella turcica and hypophysis cerebri. Key words: Sphenoid bone, Linear dimensions, Hypophysis cerebri Introduction Sella turcica is an important structure in middle cranial fossa. It is safe treatment of various pituitary disorders such as a saddle shaped concavity in the body of sphenoid bone. -

Gross Anatomy Assignment Name: Olorunfemi Peace Toluwalase Matric No: 17/Mhs01/257 Dept: Mbbs Course: Gross Anatomy of Head and Neck
GROSS ANATOMY ASSIGNMENT NAME: OLORUNFEMI PEACE TOLUWALASE MATRIC NO: 17/MHS01/257 DEPT: MBBS COURSE: GROSS ANATOMY OF HEAD AND NECK QUESTION 1 Write an essay on the carvernous sinus. The cavernous sinuses are one of several drainage pathways for the brain that sits in the middle. In addition to receiving venous drainage from the brain, it also receives tributaries from parts of the face. STRUCTURE ➢ The cavernous sinuses are 1 cm wide cavities that extend a distance of 2 cm from the most posterior aspect of the orbit to the petrous part of the temporal bone. ➢ They are bilaterally paired collections of venous plexuses that sit on either side of the sphenoid bone. ➢ Although they are not truly trabeculated cavities like the corpora cavernosa of the penis, the numerous plexuses, however, give the cavities their characteristic sponge-like appearance. ➢ The cavernous sinus is roofed by an inner layer of dura matter that continues with the diaphragma sellae that covers the superior part of the pituitary gland. The roof of the sinus also has several other attachments. ➢ Anteriorly, it attaches to the anterior and middle clinoid processes, posteriorly it attaches to the tentorium (at its attachment to the posterior clinoid process). Part of the periosteum of the greater wing of the sphenoid bone forms the floor of the sinus. ➢ The body of the sphenoid acts as the medial wall of the sinus while the lateral wall is formed from the visceral part of the dura mater. CONTENTS The cavernous sinus contains the internal carotid artery and several cranial nerves. Abducens nerve (CN VI) traverses the sinus lateral to the internal carotid artery. -

Gluteal Region-II
Gluteal Region-II Dr Garima Sehgal Associate Professor King George’s Medical University UP, Lucknow Structures in the Gluteal region • Bones & joints • Ligaments Thickest muscle • Muscles • Vessels • Nerves Thickest nerve • Bursae Learning Objectives By the end of this teaching session Gluteal region –II all the MBBS 1st year students must be able to: • Enumerate the nerves of gluteal region • Write a short note on nerves of gluteal region • Describe the location & relations of sciatic nerve in gluteal region • Enumerate the arteries of gluteal region • Write a short note on arteries of gluteal region • Enumerate the arteries taking part in trochanteric and cruciate anastomosis • Write a short note on trochanteric and cruciate anastomosis • Enumerate the structures passing through greater sciatic foramen • Enumerate the structures passing through lesser sciatic foramen • Enumerate the bursae in relation to gluteus maximus • Enumerate the structures deep to gluteus maximus • Discuss applied anatomy Nerves of Gluteal region (all nerves in gluteal region are branches of sacral plexus) Superior gluteal nerve (L4,L5, S1) Inferior gluteal nerve (L5, S1, S2) FROM DORSAL DIVISIONS Perforating cutaneous nerve (S2,S3) Nerve to quadratus femoris (L4,L5, S1) Nerve to obturator internus (L5, S1, S2) FROM VENTRAL DIVISIONS Pudendal nerve (S2,S3,S4) Sciatic nerve (L4,L5,S1,S2,S3) Posterior cutaneous nerve of thigh FROM BOTH DORSAL &VENTRAL (S1,S2) & (S2,S3) DIVISIONS 1. Superior Gluteal nerve (L4,L5,S1- dorsal division) 1 • Enters through the greater 3 sciatic foramen • Above piriformis 2 • Runs forwards between gluteus medius & gluteus minimus • SUPPLIES: 1. Gluteus medius 2. Gluteus minimus 3. Tensor fasciae latae 2. -

Craniotomy for Anterior Cranial Fossa Meningiomas: Historical Overview
Neurosurg Focus 36 (4):E14, 2014 ©AANS, 2014 Craniotomy for anterior cranial fossa meningiomas: historical overview SAUL F. MORALES-VALERO, M.D., JAMIE J. VAN GOMPEL, M.D., IOANNIS LOUMIOTIS, M.D., AND GIUSEPPE LANZINO, M.D. Department of Neurologic Surgery, Mayo Clinic, Mayo Medical School, Rochester, Minnesota The surgical treatment of meningiomas located at the base of the anterior cranial fossa is often challenging, and the evolution of the surgical strategy to resect these tumors parallels the development of craniotomy, and neurosur- gery in general, over the past century. Early successful operations to treat these tumors were pioneered by prominent figures such as Sir William Macewen and Francesco Durante. Following these early reports, Harvey Cushing made significant contributions, allowing a better understanding and treatment of meningiomas in general, but particularly those involving the anterior cranial base. Initially, large-sized unilateral or bilateral craniotomies were necessary to approach these deep-seated lesions. Technical advances such as the introduction of electrosurgery, the operating microscope, and refined microsurgical instruments allowed neurosurgeons to perform less invasive surgical proce- dures with better results. Today, a wide variety of surgical strategies, including endoscopic surgery and radiosurgery, are used to treat these tumors. In this review, the authors trace the evolution of craniotomy for anterior cranial fossa meningiomas. (http://thejns.org/doi/abs/10.3171/2014.1.FOCUS13569) KEY WORDS • intracranial meningiomas • craniotomy • history • anterior cranial fossa ENINGIOMAS of the anterior cranial fossa represent has a few distinct clinical features. However, in practice, 12%–20% of all intracranial meningiomas.5,30 this group of tumors often represents a continuum. -

The Anatomic Analysis of the Vidian Canal and the Surrounding
Braz J Otorhinolaryngol. 2019;85(2):136---143 Brazilian Journal of OTORHINOLARYNGOLOGY www.bjorl.org ORIGINAL ARTICLE The anatomic analysis of the vidian canal and the surrounding structures concerning vidian neurectomy ଝ using computed tomography scans a,∗ a b Gülay Ac¸ar , Aynur Emine C¸ic¸ekcibas¸ı , ˙Ibrahim C¸ukurova , c a d Kemal Emre Özen , Muzaffer ¸ekerS , ˙Ibrahim Güler a Necmettin Erbakan University, Meram Faculty of Medicine, Department of Anatomy, Konya, Turkey b Health Sciences University, Izmir Tepecik Trainig and Research Hospital, Department of Otolaryngology-Head and Neck Surgery, Izmir, Turkey c Katip C¸elebi University, Faculty of Medicine, Department of Anatomy, Izmir, Turkey d Selcuk University, Faculty of Medicine, Department of Radiology, Konya, Turkey Received 15 September 2017; accepted 8 November 2017 Available online 26 December 2017 KEYWORDS Abstract Intrasphenoid Introduction: The type of endoscopic approach chosen for vidian neurectomy can be specified septum; by evaluating the vidian canal and the surrounding sphenoid sinus structures. Morphometric Objective: The variations and morphometry of the vidian canal were investigated, focusing on analysis; the functional correlations between them which are crucial anatomical landmarks for preoper- Pterygoid process ative planning. pneumatization; Methods: This study was performed using paranasal multidetector computed tomography Vidian canal; images that were obtained with a section thickening of 0.625 mm of 250 adults. Vidian neurectomy Results: The distributions of 500 vidian canal variants were categorized as follows; Type 1, within the sphenoid corpus (55.6%); Type 2, partially protruding into the sphenoid sinus (34.8%); Type 3, within the sphenoid sinus (9.6%). The pneumatization of the pterygoid process is mostly seen in vidian canal Type 2 (72.4%) and Type 3 (95.8%) (p < 0.001). -
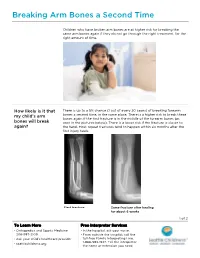
PE2812 Breaking Arm Bones a Second Time
Breaking Arm Bones a Second Time Children who have broken arm bones are at higher risk for breaking the same arm bones again if they do not go through the right treatment, for the right amount of time. How likely is it that There is up to a 5% chance (1 out of every 20 cases) of breaking forearm my child’s arm bones a second time, in the same place. There is a higher risk to break these bones again if the first fracture is in the middle of the forearm bones (as bones will break seen in the pictures below). There is a lower risk if the fracture is closer to again? the hand. Most repeat fractures tend to happen within six months after the first injury heals. First fracture Same fracture after healing for about 6 weeks 1 of 2 To Learn More Free Interpreter Services • Orthopedics and Sports Medicine • In the hospital, ask your nurse. 206-987-2109 • From outside the hospital, call the • Ask your child’s healthcare provider toll-free Family Interpreting Line, 1-866-583-1527. Tell the interpreter • seattlechildrens.org the name or extension you need. Breaking Arm Bones a Second Time How can I help my Wearing a cast for at least six weeks lowers the risk of breaking the same child lower the risk arm bones again. After wearing a cast, we recommend your child wear a brace for 4 weeks in order to protect the injured area and start improving of having a wrist movement. While your child wears a brace, we recommend they do repeated bone not participate in contact sports (e.g., soccer, football or dodge ball). -

Septation of the Sphenoid Sinus and Its Clinical Significance
1793 International Journal of Collaborative Research on Internal Medicine & Public Health Septation of the Sphenoid Sinus and its Clinical Significance Eldan Kapur 1* , Adnan Kapidžić 2, Amela Kulenović 1, Lana Sarajlić 2, Adis Šahinović 2, Maida Šahinović 3 1 Department of anatomy, Medical faculty, University of Sarajevo, Čekaluša 90, 71000 Sarajevo, Bosnia and Herzegovina 2 Clinic for otorhinolaryngology, Clinical centre University of Sarajevo, Bolnička 25, 71000 Sarajevo, Bosnia and Herzegovina 3 Department of histology and embriology, Medical faculty, University of Sarajevo, Čekaluša 90, 71000 Sarajevo, Bosnia and Herzegovina * Corresponding Author: Eldan Kapur, MD, PhD Department of anatomy, Medical faculty, University of Sarajevo, Bosnia and Herzegovina Email: [email protected] Phone: 033 66 55 49; 033 22 64 78 (ext. 136) Abstract Introduction: Sphenoid sinus is located in the body of sphenoid, closed with a thin plate of bone tissue that separates it from the important structures such as the optic nerve, optic chiasm, cavernous sinus, pituitary gland, and internal carotid artery. It is divided by one or more vertical septa that are often asymmetric. Because of its location and the relationships with important neurovascular and glandular structures, sphenoid sinus represents a great diagnostic and therapeutic challenge. Aim: The aim of this study was to assess the septation of the sphenoid sinus and relationship between the number and position of septa and internal carotid artery in the adult BH population. Participants and Methods: A retrospective study of the CT analysis of the paranasal sinuses in 200 patients (104 male, 96 female) were performed using Siemens Somatom Art with the following parameters: 130 mAs: 120 kV, Slice: 3 mm. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Perinate and Eggs of a Giant Caenagnathid Dinosaur from the Late Cretaceous of Central China
ARTICLE Received 29 Jul 2016 | Accepted 15 Feb 2017 | Published 9 May 2017 DOI: 10.1038/ncomms14952 OPEN Perinate and eggs of a giant caenagnathid dinosaur from the Late Cretaceous of central China Hanyong Pu1, Darla K. Zelenitsky2, Junchang Lu¨3, Philip J. Currie4, Kenneth Carpenter5,LiXu1, Eva B. Koppelhus4, Songhai Jia1, Le Xiao1, Huali Chuang1, Tianran Li1, Martin Kundra´t6 & Caizhi Shen3 The abundance of dinosaur eggs in Upper Cretaceous strata of Henan Province, China led to the collection and export of countless such fossils. One of these specimens, recently repatriated to China, is a partial clutch of large dinosaur eggs (Macroelongatoolithus) with a closely associated small theropod skeleton. Here we identify the specimen as an embryo and eggs of a new, large caenagnathid oviraptorosaur, Beibeilong sinensis. This specimen is the first known association between skeletal remains and eggs of caenagnathids. Caenagnathids and oviraptorids share similarities in their eggs and clutches, although the eggs of Beibeilong are significantly larger than those of oviraptorids and indicate an adult body size comparable to a gigantic caenagnathid. An abundance of Macroelongatoolithus eggs reported from Asia and North America contrasts with the dearth of giant caenagnathid skeletal remains. Regardless, the large caenagnathid-Macroelongatoolithus association revealed here suggests these dinosaurs were relatively common during the early Late Cretaceous. 1 Henan Geological Museum, Zhengzhou 450016, China. 2 Department of Geoscience, University of Calgary, Calgary, Alberta, Canada T2N 1N4. 3 Institute of Geology, Chinese Academy of Geological Sciences, Beijing 100037, China. 4 Department of Biological Sciences, University of Alberta, Edmonton, Alberta, Canada T6G 2E9. 5 Prehistoric Museum, Utah State University, 155 East Main Street, Price, Utah 84501, USA.