Pollination System Transitions in Ruellia (Acanthaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paynes Creek Historic State Park
Paynes Creek Historic State Park Approved Plan Unit Management Plan STATE OF FLORIDA DEPARTMENT OF ENVIRONMENTAL PROTECTION Division of Recreation and Parks December 16, 2016 TABLE OF CONTENTS INTRODUCTION ...................................................................................1 PURPOSE AND SIGNIFICANCE OF THE PARK ....................................... 1 Park Significance ................................................................................1 PURPOSE AND SCOPE OF THE PLAN..................................................... 2 MANAGEMENT PROGRAM OVERVIEW ................................................... 8 Management Authority and Responsibility .............................................. 8 Park Management Goals ...................................................................... 8 Management Coordination ................................................................... 9 Public Participation ..............................................................................9 Other Designations .............................................................................9 RESOURCE MANAGEMENT COMPONENT INTRODUCTION ................................................................................. 11 RESOURCE DESCRIPTION AND ASSESSMENT..................................... 12 Natural Resources ............................................................................. 12 Topography .................................................................................. 12 Geology ...................................................................................... -

Natural Heritage Program List of Rare Plant Species of North Carolina 2016
Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Revised February 24, 2017 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. -

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Natural Communities of Michigan: Classification and Description
Natural Communities of Michigan: Classification and Description Prepared by: Michael A. Kost, Dennis A. Albert, Joshua G. Cohen, Bradford S. Slaughter, Rebecca K. Schillo, Christopher R. Weber, and Kim A. Chapman Michigan Natural Features Inventory P.O. Box 13036 Lansing, MI 48901-3036 For: Michigan Department of Natural Resources Wildlife Division and Forest, Mineral and Fire Management Division September 30, 2007 Report Number 2007-21 Version 1.2 Last Updated: July 9, 2010 Suggested Citation: Kost, M.A., D.A. Albert, J.G. Cohen, B.S. Slaughter, R.K. Schillo, C.R. Weber, and K.A. Chapman. 2007. Natural Communities of Michigan: Classification and Description. Michigan Natural Features Inventory, Report Number 2007-21, Lansing, MI. 314 pp. Copyright 2007 Michigan State University Board of Trustees. Michigan State University Extension programs and materials are open to all without regard to race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, marital status or family status. Cover photos: Top left, Dry Sand Prairie at Indian Lake, Newaygo County (M. Kost); top right, Limestone Bedrock Lakeshore, Summer Island, Delta County (J. Cohen); lower left, Muskeg, Luce County (J. Cohen); and lower right, Mesic Northern Forest as a matrix natural community, Porcupine Mountains Wilderness State Park, Ontonagon County (M. Kost). Acknowledgements We thank the Michigan Department of Natural Resources Wildlife Division and Forest, Mineral, and Fire Management Division for funding this effort to classify and describe the natural communities of Michigan. This work relied heavily on data collected by many present and former Michigan Natural Features Inventory (MNFI) field scientists and collaborators, including members of the Michigan Natural Areas Council. -

A Compilation and Analysis of Food Plants Utilization of Sri Lankan Butterfly Larvae (Papilionoidea)
MAJOR ARTICLE TAPROBANICA, ISSN 1800–427X. August, 2014. Vol. 06, No. 02: pp. 110–131, pls. 12, 13. © Research Center for Climate Change, University of Indonesia, Depok, Indonesia & Taprobanica Private Limited, Homagama, Sri Lanka http://www.sljol.info/index.php/tapro A COMPILATION AND ANALYSIS OF FOOD PLANTS UTILIZATION OF SRI LANKAN BUTTERFLY LARVAE (PAPILIONOIDEA) Section Editors: Jeffrey Miller & James L. Reveal Submitted: 08 Dec. 2013, Accepted: 15 Mar. 2014 H. D. Jayasinghe1,2, S. S. Rajapaksha1, C. de Alwis1 1Butterfly Conservation Society of Sri Lanka, 762/A, Yatihena, Malwana, Sri Lanka 2 E-mail: [email protected] Abstract Larval food plants (LFPs) of Sri Lankan butterflies are poorly documented in the historical literature and there is a great need to identify LFPs in conservation perspectives. Therefore, the current study was designed and carried out during the past decade. A list of LFPs for 207 butterfly species (Super family Papilionoidea) of Sri Lanka is presented based on local studies and includes 785 plant-butterfly combinations and 480 plant species. Many of these combinations are reported for the first time in Sri Lanka. The impact of introducing new plants on the dynamics of abundance and distribution of butterflies, the possibility of butterflies being pests on crops, and observations of LFPs of rare butterfly species, are discussed. This information is crucial for the conservation management of the butterfly fauna in Sri Lanka. Key words: conservation, crops, larval food plants (LFPs), pests, plant-butterfly combination. Introduction Butterflies go through complete metamorphosis 1949). As all herbivorous insects show some and have two stages of food consumtion. -

10 Easy Wildflowers for Butterflies and Bees Tips and Terms
10 Easy Wildflowers for Butterflies and Bees Tips and Terms Selection Glossary of helpful terms It may take a while to understand your landscape’s soil and drainage conditions. If Anther: pollen-bearing part of the stamen your wildflowers don’t succeed, try again, maybe with different species. Remember, Axil: upper angle between the stem and success depends on using the right plant in the right place. leaf or other plant part Water Basal: forming or attached at the base Water plants thoroughly when planting, then water as needed until they are established Bract: modified leaf at the base of a flower and putting out new foliage. Once plants are established, irrigation should be needed Calyx: collective term for the sepals of a only during extended dry periods. Learn to recognize when plants look wilted and flower; typically a whorl that encloses water them then. Over-irrigation can cause fungus and rot, which can kill your the petals and protects the flower bud wildflowers. It can also cause them to grow too quickly, becoming more susceptible to pests and diseases, or too tall, requiring staking. Corolla: collective term for the petals of a flower Fertilizer Corona: petal-like structures arising from Native wildflowers should not need fertilizer. Applying fertilizer can produce plants that the corolla of some flowers to form a grow too quickly, which can lead them to become pest and disease prone, and too tall, crownlike ring requiring staking. Fertilizing also encourages weeds, which can easily out-compete Cultivar: horticultural variety of a wildflowers. naturally occurring species produced in cultivation by selective breeding Sustaining wildflowers If you want wildflowers to persist on their own in your landscape, you’ll need to allow for Deciduous: seasonal shedding of leaves; self-seeding, especially for annual or short-lived species. -

Sinopsis De La Familia Acanthaceae En El Perú
Revista Forestal del Perú, 34 (1): 21 - 40, (2019) ISSN 0556-6592 (Versión impresa) / ISSN 2523-1855 (Versión electrónica) © Facultad de Ciencias Forestales, Universidad Nacional Agraria La Molina, Lima-Perú DOI: http://dx.doi.org/10.21704/rfp.v34i1.1282 Sinopsis de la familia Acanthaceae en el Perú A synopsis of the family Acanthaceae in Peru Rosa M. Villanueva-Espinoza1, * y Florangel M. Condo1 Recibido: 03 marzo 2019 | Aceptado: 28 abril 2019 | Publicado en línea: 30 junio 2019 Citación: Villanueva-Espinoza, RM; Condo, FM. 2019. Sinopsis de la familia Acanthaceae en el Perú. Revista Forestal del Perú 34(1): 21-40. DOI: http://dx.doi.org/10.21704/rfp.v34i1.1282 Resumen La familia Acanthaceae en el Perú solo ha sido revisada por Brako y Zarucchi en 1993, desde en- tonces, se ha generado nueva información sobre esta familia. El presente trabajo es una sinopsis de la familia Acanthaceae donde cuatro subfamilias (incluyendo Avicennioideae) y 38 géneros son reconocidos. El tratamiento de cada género incluye su distribución geográfica, número de especies, endemismo y carácteres diagnósticos. Un total de ocho nombres (Juruasia Lindau, Lo phostachys Pohl, Teliostachya Nees, Streblacanthus Kuntze, Blechum P. Browne, Habracanthus Nees, Cylindrosolenium Lindau, Hansteinia Oerst.) son subordinados como sinónimos y, tres especies endémicas son adicionadas para el país. Palabras clave: Acanthaceae, actualización, morfología, Perú, taxonomía Abstract The family Acanthaceae in Peru has just been reviewed by Brako and Zarruchi in 1993, since then, new information about this family has been generated. The present work is a synopsis of family Acanthaceae where four subfamilies (includying Avicennioideae) and 38 genera are recognized. -

Cocoa Beach Maritime Hammock Preserve Management Plan
MANAGEMENT PLAN Cocoa Beach’s Maritime Hammock Preserve City of Cocoa Beach, Florida Florida Communities Trust Project No. 03 – 035 –FF3 Adopted March 18, 2004 TABLE OF CONTENTS SECTION PAGE I. Introduction ……………………………………………………………. 1 II. Purpose …………………………………………………………….……. 2 a. Future Uses ………….………………………………….…….…… 2 b. Management Objectives ………………………………………….... 2 c. Major Comprehensive Plan Directives ………………………..….... 2 III. Site Development and Improvement ………………………………… 3 a. Existing Physical Improvements ……….…………………………. 3 b. Proposed Physical Improvements…………………………………… 3 c. Wetland Buffer ………...………….………………………………… 4 d. Acknowledgment Sign …………………………………..………… 4 e. Parking ………………………….………………………………… 5 f. Stormwater Facilities …………….………………………………… 5 g. Hazard Mitigation ………………………………………………… 5 h. Permits ………………………….………………………………… 5 i. Easements, Concessions, and Leases …………………………..… 5 IV. Natural Resources ……………………………………………..……… 6 a. Natural Communities ………………………..……………………. 6 b. Listed Animal Species ………………………….…………….……. 7 c. Listed Plant Species …………………………..…………………... 8 d. Inventory of the Natural Communities ………………..………….... 10 e. Water Quality …………..………………………….…..…………... 10 f. Unique Geological Features ………………………………………. 10 g. Trail Network ………………………………….…..………..……... 10 h. Greenways ………………………………….…..……………..……. 11 i Adopted March 18, 2004 V. Resources Enhancement …………………………..…………………… 11 a. Upland Restoration ………………………..………………………. 11 b. Wetland Restoration ………………………….…………….………. 13 c. Invasive Exotic Plants …………………………..…………………... 13 d. Feral -
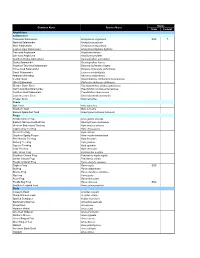
St. Joseph Bay Native Species List
Status Common Name Species Name State Federal Amphibians Salamanders Flatwoods Salamander Ambystoma cingulatum SSC T Marbled Salamander Ambystoma opacum Mole Salamander Ambystoma talpoideum Eastern Tiger Salamander Ambystoma tigrinum tigrinum Two-toed Amphiuma Amphiuma means One-toed Amphiuma Amphiuma pholeter Southern Dusky Salamander Desmognathus auriculatus Dusky Salamander Desmognathus fuscus Southern Two-lined Salamander Eurycea bislineata cirrigera Three-lined Salamander Eurycea longicauda guttolineata Dwarf Salamander Eurycea quadridigitata Alabama Waterdog Necturus alabamensis Central Newt Notophthalmus viridescens louisianensis Slimy Salamander Plethodon glutinosus glutinosus Slender Dwarf Siren Pseudobranchus striatus spheniscus Gulf Coast Mud Salamander Pseudotriton montanus flavissimus Southern Red Salamander Pseudotriton ruber vioscai Eastern Lesser Siren Siren intermedia intermedia Greater Siren Siren lacertina Toads Oak Toad Bufo quercicus Southern Toad Bufo terrestris Eastern Spadefoot Toad Scaphiopus holbrooki holbrooki Frogs Florida Cricket Frog Acris gryllus dorsalis Eastern Narrow-mouthed Frog Gastrophryne carolinensis Western Bird-voiced Treefrog Hyla avivoca avivoca Cope's Gray Treefrog Hyla chrysoscelis Green Treefrog Hyla cinerea Southern Spring Peeper Hyla crucifer bartramiana Pine Woods Treefrog Hyla femoralis Barking Treefrog Hyla gratiosa Squirrel Treefrog Hyla squirella Gray Treefrog Hyla versicolor Little Grass Frog Limnaoedus ocularis Southern Chorus Frog Pseudacris nigrita nigrita Ornate Chorus Frog Pseudacris -

Phytochemical Studies and Antibacterial Activities of Ruellia Tuberosa L
University Journal of Creativity and Innovative Research 2020 Vol-01 Issue-01 Phytochemical Studies and Antibacterial Activities of Ruellia tuberosa L. Khin Nwe Than Khin Myint Maw Yadanabon University Mandalay University of Distance Education [email protected] [email protected] Abstract antidote activity. These plants are used to cure antidote for snake-bite, centipede and gecko-bite according to [2]. The medicinal plant Ruellia tuberosa L. of The preliminary phytochemical investigation was Acanthaceae family is a tropical plant widely carried out by ethanol extracts for study of plants distributed in South East Asia. In phytochemical containing chemical constituents (alkaloid, steroid, investigation, 10 compounds were studied. Among them, flavonoid, glycoside, tannin, reducing sugar, phenolic glycoside, alkaloid, reducing sugar, terpene, phenolic compound, saponin, polyphenol and terpenoid). compound, polyphenol, saponin and tannin were Antibacterial activities were also made with various present but flavonoid and steroid were absent in the extract (ethanol, ethyl- acetate and n-hexane) of the powder of study plant. Moreover, the antibacterial whole plant of R. tuberosa L. by using agar well activity were carried out by using 95% ethanol, ethyl- diffusion method against six different types of test acetate and n-hexane. Ethanol extract, n-hexane extract organisms. The present research aimed to investigate the and ethyl-acetate extract were obtained the best chemical composition of Ruellia tuberosa L. as well as inhibition zone diameter (0.7 cm) on Salmonella typhi. to determine the antibacterial activity. The inhibition zone of ethanol extract showed the most effective on Staphylococcus aureus (0.8 cm) and less 2. Materials and Methods inhibition zone showed on Pseudomonas aeruginosa (0.3 cm). -

ACANTHACEAE 爵床科 Jue Chuang Ke Hu Jiaqi (胡嘉琪 Hu Chia-Chi)1, Deng Yunfei (邓云飞)2; John R
ACANTHACEAE 爵床科 jue chuang ke Hu Jiaqi (胡嘉琪 Hu Chia-chi)1, Deng Yunfei (邓云飞)2; John R. I. Wood3, Thomas F. Daniel4 Prostrate, erect, or rarely climbing herbs (annual or perennial), subshrubs, shrubs, or rarely small trees, usually with cystoliths (except in following Chinese genera: Acanthus, Blepharis, Nelsonia, Ophiorrhiziphyllon, Staurogyne, and Thunbergia), isophyllous (leaf pairs of equal size at each node) or anisophyllous (leaf pairs of unequal size at each node). Branches decussate, terete to angular in cross-section, nodes often swollen, sometimes spinose with spines derived from reduced leaves, bracts, and/or bracteoles. Stipules absent. Leaves opposite [rarely alternate or whorled]; leaf blade margin entire, sinuate, crenate, dentate, or rarely pinnatifid. Inflo- rescences terminal or axillary spikes, racemes, panicles, or dense clusters, rarely of solitary flowers; bracts 1 per flower or dichasial cluster, large and brightly colored or minute and green, sometimes becoming spinose; bracteoles present or rarely absent, usually 2 per flower. Flowers sessile or pedicellate, bisexual, zygomorphic to subactinomorphic. Calyx synsepalous (at least basally), usually 4- or 5-lobed, rarely (Thunbergia) reduced to an entire cupular ring or 10–20-lobed. Corolla sympetalous, sometimes resupinate 180º by twisting of corolla tube; tube cylindric or funnelform; limb subactinomorphic (i.e., subequally 5-lobed) or zygomorphic (either 2- lipped with upper lip subentire to 2-lobed and lower lip 3-lobed, or rarely 1-lipped with 3 lobes); lobes ascending or descending cochlear, quincuncial, contorted, or open in bud. Stamens epipetalous, included in or exserted from corolla tube, 2 or 4 and didyna- mous; filaments distinct, connate in pairs, or monadelphous basally via a sheath (Strobilanthes); anthers with 1 or 2 thecae; thecae parallel to perpendicular, equally inserted to superposed, spherical to linear, base muticous or spurred, usually longitudinally dehis- cent; staminodes 0–3, consisting of minute projections or sterile filaments. -

Botanist Interior 43.1
196 THE MICHIGAN BOTANIST Vol. 44 NEW RECORDS FOR RUELLIA HUMILIS NUTTALL (ACANTHACEAE) IN WISCONSIN Thomas L. Eddy 426 Walker Avenue Green Lake, WI 54941 [email protected] The genus Ruellia Linnaeus is represented by four species in northeastern U.S. and adjacent Canada (Gleason and Cronquist 1991). Of these, Ruellia hu- milis Nuttall is reported to occur in 25 states in the U.S. The plant is listed on state threatened (T) and endangered (E) species lists in Maryland (E), Michigan (T), North Carolina (T) and Pennsylvania (E). In Wisconsin R. humilis is endan- gered (WDNR, 2005), known from six counties in the two southernmost-tiers of the state (Crawford, Dane, Grant, Portage, Rock, and Walworth) (Wisflora, 2005). Two recent collections of R. humilis in Outagamie and Winnebago coun- ties have extended the known range distribution to northeastern Wisconsin. Ruellia humilis is a perennial forb that goes by numerous common names: fringe-leaf ruellia, hairy ruellia, hairy wild petunia, and wild petunia. Two vari- eties of R. humilis are recognized: var. calvescens Fern. of the Appalachian re- gion and var. humilis (subject of this report), which is the common and wide- spread variety with densely hairy internodes, calyx lobes, and leaf veins and margins. Ruellia humilis var. humilis inhabits prairies and dry upland woods, ranging from Pennsylvania to northern Indiana, southeastern Minnesota, Nebraska, south to western North Carolina, Alabama, and Texas (Gleason & Cronquist, 1991). In Wisconsin R. humilis is quite rare. It flowers near the end of June to mid- September and fruits from the end of July to September.