Amphibian Diversity in Amazonian Flooded Forests of Peru
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

01-Marty 148.Indd
Copy proofs - 2-12-2013 Bull. Soc. Herp. Fr. (2013) 148 : 419-424 On the occurrence of Dendropsophus leali (Bokermann, 1964) (Anura; Hylidae) in French Guiana by Christian MARTY (1)*, Michael LEBAILLY (2), Philippe GAUCHER (3), Olivier ToSTAIN (4), Maël DEWYNTER (5), Michel BLANC (6) & Antoine FOUQUET (3). (1) Impasse Jean Galot, 97354 Montjoly, Guyane française [email protected] (2) Health Center, 97316, Antécum Pata, Guyane française (3) CNRS Guyane USR 3456, Immeuble Le Relais, 2 avenue Gustave Charlery, 97300 Cayenne, Guyane française (4) Ecobios, BP 44, 97321, Cayenne CEDEX , Guyane française (5) Biotope, Agence Amazonie-Caraïbes, 30 domaine de Montabo, Lotissement Ribal, 97300 Cayenne, Guyane française (6) Pointe Maripa, RN2/PK35, Roura, Guyane française Summary – Dendropsophus leali is a small Amazonian tree frog occurring in Brazil, Peru, Bolivia and Colombia where it mostly inhabits patches of open habitat and disturbed forest. We herein report five new records of this species from French Guiana extending its range 650 km to the north-east and sug- gesting that D. leali could be much more widely distributed in Amazonia than previously thought. The origin of such a disjunct distribution pattern probably lies in historical fluctuations of the forest cover during the late Tertiary and the Quaternary. Poor understanding of Amazonian species distribution still impedes comprehensive investigation of the processes that have shaped Amazonian megabiodiversity. Key-words: Dendropsophus leali, Anura, Hylidae, distribution, French Guiana. Résumé – À propos de la présence de Dendropsophus leali (Bokermann, 1964) (Anura ; Hylidae) en Guyane française. Dendropsophus leali est une rainette de petite taille présente au Brésil, au Pérou, en Bolivie et en Colombie où elle occupe principalement des habitats ouverts ou des forêts perturbées. -

Catalogue of the Amphibians of Venezuela: Illustrated and Annotated Species List, Distribution, and Conservation 1,2César L
Mannophryne vulcano, Male carrying tadpoles. El Ávila (Parque Nacional Guairarepano), Distrito Federal. Photo: Jose Vieira. We want to dedicate this work to some outstanding individuals who encouraged us, directly or indirectly, and are no longer with us. They were colleagues and close friends, and their friendship will remain for years to come. César Molina Rodríguez (1960–2015) Erik Arrieta Márquez (1978–2008) Jose Ayarzagüena Sanz (1952–2011) Saúl Gutiérrez Eljuri (1960–2012) Juan Rivero (1923–2014) Luis Scott (1948–2011) Marco Natera Mumaw (1972–2010) Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [Special Section]: 1–198 (e180). Catalogue of the amphibians of Venezuela: Illustrated and annotated species list, distribution, and conservation 1,2César L. Barrio-Amorós, 3,4Fernando J. M. Rojas-Runjaic, and 5J. Celsa Señaris 1Fundación AndígenA, Apartado Postal 210, Mérida, VENEZUELA 2Current address: Doc Frog Expeditions, Uvita de Osa, COSTA RICA 3Fundación La Salle de Ciencias Naturales, Museo de Historia Natural La Salle, Apartado Postal 1930, Caracas 1010-A, VENEZUELA 4Current address: Pontifícia Universidade Católica do Río Grande do Sul (PUCRS), Laboratório de Sistemática de Vertebrados, Av. Ipiranga 6681, Porto Alegre, RS 90619–900, BRAZIL 5Instituto Venezolano de Investigaciones Científicas, Altos de Pipe, apartado 20632, Caracas 1020, VENEZUELA Abstract.—Presented is an annotated checklist of the amphibians of Venezuela, current as of December 2018. The last comprehensive list (Barrio-Amorós 2009c) included a total of 333 species, while the current catalogue lists 387 species (370 anurans, 10 caecilians, and seven salamanders), including 28 species not yet described or properly identified. Fifty species and four genera are added to the previous list, 25 species are deleted, and 47 experienced nomenclatural changes. -

Nototriton Nelsoni Is a Moss Salamander Endemic to Cloud Forest in Refugio De Vida Silvestre Texíguat, Located in the Departments of Atlántida and Yoro, Honduras
Nototriton nelsoni is a moss salamander endemic to cloud forest in Refugio de Vida Silvestre Texíguat, located in the departments of Atlántida and Yoro, Honduras. This cryptic species long was confused with N. barbouri, a morphologically similar species now considered endemic to the Sierra de Sulaco in the southern part of the department of Yoro. Like many of its congeners, N. nelsoni rarely is observed in the wild, and is known from just five specimens. Pictured here is the holotype of N. nelsoni, collected above La Liberación in Refugio de Vida Silvestre Texíguat at an elevation of 1,420 m. This salamander is one of many herpetofaunal species endemic to the Cordillera Nombre de Dios. ' © Josiah H. Townsend 909 www.mesoamericanherpetology.com www.eaglemountainpublishing.com Amphibians of the Cordillera Nombre de Dios, Honduras: COI barcoding suggests underestimated taxonomic richness in a threatened endemic fauna JOSIAH H. TOWNSEND1 AND LARRY DAVID WILSON2 1Department of Biology, Indiana University of Pennsylvania, Indiana, Pennsylvania 15705–1081, United States. E-mail: [email protected] (Corresponding author) 2Centro Zamorano de Biodiversidad, Escuela Agrícola Panamericana Zamorano, Departamento de Francisco Morazán, Honduras; 16010 SW 207th Avenue, Miami, Florida 33187-1067, United States. E-mail: [email protected] ABSTRACT: The Cordillera Nombre de Dios is a chain of mountains along the northern coast of Honduras that harbors a high degree of herpetofaunal endemism. We present a preliminary barcode reference library of amphibians from the Cordillera Nombre de Dios, based on sampling at 10 sites from 2008 to 2013. We sequenced 187 samples of 21 nominal taxa for the barcoding locus cytochrome oxidase subunit I (COI), and recovered 28 well-differentiated clades. -

Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca
Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organizations. Published by: IUCN, Gland, Switzerland Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Lamoreux, J. F., McKnight, M. W., and R. Cabrera Hernandez (2015). Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca. Gland, Switzerland: IUCN. xxiv + 320pp. ISBN: 978-2-8317-1717-3 DOI: 10.2305/IUCN.CH.2015.SSC-OP.53.en Cover photographs: Totontepec landscape; new Plectrohyla species, Ixalotriton niger, Concepción Pápalo, Thorius minutissimus, Craugastor pozo (panels, left to right) Back cover photograph: Collecting in Chamula, Chiapas Photo credits: The cover photographs were taken by the authors under grant agreements with the two main project funders: NGS and CEPF. -

Furness, Mcdiarmid, Heyer, Zug.Indd
south american Journal of Herpetology, 5(1), 2010, 13-29 © 2010 brazilian society of herpetology Oviduct MOdificatiOns in fOaM-nesting frOgs, with eMphasis On the genus LeptodactyLus (aMphibia, LeptOdactyLidae) Andrew I. Furness1, roy w. McdIArMId2, w. ronAld Heyer3,5, And GeorGe r. ZuG4 1 department of Biology, university of california, Riverside, ca 92501, usa. e‑mail: [email protected] 2 us Geological survey, patuxent Wildlife Research center, National Museum of Natural History, MRc 111, po Box 37012, smithsonian Institution, Washington, dc 20013‑7012, usa. e‑mail: [email protected] 3 National Museum of Natural History, MRc 162, po Box 37012, smithsonian Institution, Washington, dc 20013‑7012. e‑mail: [email protected] 4 National Museum of Natural History, MRc 162, po Box 37012, smithsonian Institution, Washington, dc 20013‑7012. e‑mail: [email protected] 5 corresponding author. AbstrAct. various species of frogs produce foam nests that hold their eggs during development. we examined the external morphology and histology of structures associated with foam nest production in frogs of the genus Leptodactylus and a few other taxa. we found that the posterior convolutions of the oviducts in all mature female foam-nesting frogs that we examined were enlarged and compressed into globular structures. this organ-like portion of the oviduct has been called a “foam gland” and these structures almost certainly produce the secretion that is beaten by rhythmic limb movements into foam that forms the nest. however, the label “foam gland” is a misnomer because the structures are simply enlarged and tightly folded regions of the pars convoluta of the oviduct, rather than a separate structure; we suggest the name pars convoluta dilata (pcd) for this feature. -

The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom
lopmen ve ta e l B Williamson, Cell Dev Biol 2012, 1:1 D io & l l o l g DOI: 10.4172/2168-9296.1000101 e y C Cell & Developmental Biology ISSN: 2168-9296 Research Article Open Access The Origins of Chordate Larvae Donald I Williamson* Marine Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom Abstract The larval transfer hypothesis states that larvae originated as adults in other taxa and their genomes were transferred by hybridization. It contests the view that larvae and corresponding adults evolved from common ancestors. The present paper reviews the life histories of chordates, and it interprets them in terms of the larval transfer hypothesis. It is the first paper to apply the hypothesis to craniates. I claim that the larvae of tunicates were acquired from adult larvaceans, the larvae of lampreys from adult cephalochordates, the larvae of lungfishes from adult craniate tadpoles, and the larvae of ray-finned fishes from other ray-finned fishes in different families. The occurrence of larvae in some fishes and their absence in others is correlated with reproductive behavior. Adult amphibians evolved from adult fishes, but larval amphibians did not evolve from either adult or larval fishes. I submit that [1] early amphibians had no larvae and that several families of urodeles and one subfamily of anurans have retained direct development, [2] the tadpole larvae of anurans and urodeles were acquired separately from different Mesozoic adult tadpoles, and [3] the post-tadpole larvae of salamanders were acquired from adults of other urodeles. Reptiles, birds and mammals probably evolved from amphibians that never acquired larvae. -
For Review Only
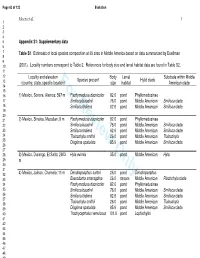
Page 63 of 123 Evolution Moen et al. 1 1 2 3 4 5 Appendix S1: Supplementary data 6 7 Table S1 . Estimates of local species composition at 39 sites in Middle America based on data summarized by Duellman 8 9 10 (2001). Locality numbers correspond to Table 2. References for body size and larval habitat data are found in Table S2. 11 12 Locality and elevation Body Larval Subclade within Middle Species present Hylid clade 13 (country, state, specific location)For Reviewsize Only habitat American clade 14 15 16 1) Mexico, Sonora, Alamos; 597 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 17 Smilisca baudinii 76.0 pond Middle American Smilisca clade 18 Smilisca fodiens 62.6 pond Middle American Smilisca clade 19 20 21 2) Mexico, Sinaloa, Mazatlan; 9 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 22 Smilisca baudinii 76.0 pond Middle American Smilisca clade 23 Smilisca fodiens 62.6 pond Middle American Smilisca clade 24 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 25 Diaglena spatulata 85.9 pond Middle American Smilisca clade 26 27 28 3) Mexico, Durango, El Salto; 2603 Hyla eximia 35.0 pond Middle American Hyla 29 m 30 31 32 4) Mexico, Jalisco, Chamela; 11 m Dendropsophus sartori 26.0 pond Dendropsophus 33 Exerodonta smaragdina 26.0 stream Middle American Plectrohyla clade 34 Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 35 Smilisca baudinii 76.0 pond Middle American Smilisca clade 36 Smilisca fodiens 62.6 pond Middle American Smilisca clade 37 38 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 39 Diaglena spatulata 85.9 pond Middle American Smilisca clade 40 Trachycephalus venulosus 101.0 pond Lophiohylini 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 Evolution Page 64 of 123 Moen et al. -

Mannophryne Olmonae) Catherine G
The College of Wooster Libraries Open Works Senior Independent Study Theses 2014 A Not-So-Silent Spring: The mpI acts of Traffic Noise on Call Features of The loB ody Bay Poison Frog (Mannophryne olmonae) Catherine G. Clemmens The College of Wooster, [email protected] Follow this and additional works at: https://openworks.wooster.edu/independentstudy Part of the Other Environmental Sciences Commons Recommended Citation Clemmens, Catherine G., "A Not-So-Silent Spring: The mpI acts of Traffico N ise on Call Features of The loodyB Bay Poison Frog (Mannophryne olmonae)" (2014). Senior Independent Study Theses. Paper 5783. https://openworks.wooster.edu/independentstudy/5783 This Senior Independent Study Thesis Exemplar is brought to you by Open Works, a service of The oC llege of Wooster Libraries. It has been accepted for inclusion in Senior Independent Study Theses by an authorized administrator of Open Works. For more information, please contact [email protected]. © Copyright 2014 Catherine G. Clemmens A NOT-SO-SILENT SPRING: THE IMPACTS OF TRAFFIC NOISE ON CALL FEATURES OF THE BLOODY BAY POISON FROG (MANNOPHRYNE OLMONAE) DEPARTMENT OF BIOLOGY INDEPENDENT STUDY THESIS Catherine Grace Clemmens Adviser: Richard Lehtinen Submitted in Partial Fulfillment of the Requirement for Independent Study Thesis in Biology at the COLLEGE OF WOOSTER 2014 TABLE OF CONTENTS I. ABSTRACT II. INTRODUCTION…………………………………………...............…...........1 a. Behavioral Effects of Anthropogenic Noise……………………….........2 b. Effects of Anthropogenic Noise on Frog Vocalization………………....6 c. Why Should We Care? The Importance of Calling for Frogs..................8 d. Color as a Mode of Communication……………………………….…..11 e. Biology of the Bloody Bay Poison Frog (Mannophryne olmonae)…...13 III. -

Species Diversity and Conservation Status of Amphibians in Madre De Dios, Southern Peru
Herpetological Conservation and Biology 4(1):14-29 Submitted: 18 December 2007; Accepted: 4 August 2008 SPECIES DIVERSITY AND CONSERVATION STATUS OF AMPHIBIANS IN MADRE DE DIOS, SOUTHERN PERU 1,2 3 4,5 RUDOLF VON MAY , KAREN SIU-TING , JENNIFER M. JACOBS , MARGARITA MEDINA- 3 6 3,7 1 MÜLLER , GIUSEPPE GAGLIARDI , LILY O. RODRÍGUEZ , AND MAUREEN A. DONNELLY 1 Department of Biological Sciences, Florida International University, 11200 SW 8th Street, OE-167, Miami, Florida 33199, USA 2 Corresponding author, e-mail: [email protected] 3 Departamento de Herpetología, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Avenida Arenales 1256, Lima 11, Perú 4 Department of Biology, San Francisco State University, 1600 Holloway Avenue, San Francisco, California 94132, USA 5 Department of Entomology, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, California 94118, USA 6 Departamento de Herpetología, Museo de Zoología de la Universidad Nacional de la Amazonía Peruana, Pebas 5ta cuadra, Iquitos, Perú 7 Programa de Desarrollo Rural Sostenible, Cooperación Técnica Alemana – GTZ, Calle Diecisiete 355, Lima 27, Perú ABSTRACT.—This study focuses on amphibian species diversity in the lowland Amazonian rainforest of southern Peru, and on the importance of protected and non-protected areas for maintaining amphibian assemblages in this region. We compared species lists from nine sites in the Madre de Dios region, five of which are in nationally recognized protected areas and four are outside the country’s protected area system. Los Amigos, occurring outside the protected area system, is the most species-rich locality included in our comparison. -

Thermal Adaptation of Amphibians in Tropical Mountains
Thermal adaptation of amphibians in tropical mountains. Consequences of global warming Adaptaciones térmicas de anfibios en montañas tropicales: consecuencias del calentamiento global Adaptacions tèrmiques d'amfibis en muntanyes tropicals: conseqüències de l'escalfament global Pol Pintanel Costa ADVERTIMENT. La consulta d’aquesta tesi queda condicionada a l’acceptació de les següents condicions d'ús: La difusió d’aquesta tesi per mitjà del servei TDX (www.tdx.cat) i a través del Dipòsit Digital de la UB (diposit.ub.edu) ha estat autoritzada pels titulars dels drets de propietat intel·lectual únicament per a usos privats emmarcats en activitats d’investigació i docència. No s’autoritza la seva reproducció amb finalitats de lucre ni la seva difusió i posada a disposició des d’un lloc aliè al servei TDX ni al Dipòsit Digital de la UB. No s’autoritza la presentació del seu contingut en una finestra o marc aliè a TDX o al Dipòsit Digital de la UB (framing). Aquesta reserva de drets afecta tant al resum de presentació de la tesi com als seus continguts. En la utilització o cita de parts de la tesi és obligat indicar el nom de la persona autora. ADVERTENCIA. La consulta de esta tesis queda condicionada a la aceptación de las siguientes condiciones de uso: La difusión de esta tesis por medio del servicio TDR (www.tdx.cat) y a través del Repositorio Digital de la UB (diposit.ub.edu) ha sido autorizada por los titulares de los derechos de propiedad intelectual únicamente para usos privados enmarcados en actividades de investigación y docencia. -

Etar a Área De Distribuição Geográfica De Anfíbios Na Amazônia
Universidade Federal do Amapá Pró-Reitoria de Pesquisa e Pós-Graduação Programa de Pós-Graduação em Biodiversidade Tropical Mestrado e Doutorado UNIFAP / EMBRAPA-AP / IEPA / CI-Brasil YURI BRENO DA SILVA E SILVA COMO A EXPANSÃO DE HIDRELÉTRICAS, PERDA FLORESTAL E MUDANÇAS CLIMÁTICAS AMEAÇAM A ÁREA DE DISTRIBUIÇÃO DE ANFÍBIOS NA AMAZÔNIA BRASILEIRA MACAPÁ, AP 2017 YURI BRENO DA SILVA E SILVA COMO A EXPANSÃO DE HIDRE LÉTRICAS, PERDA FLORESTAL E MUDANÇAS CLIMÁTICAS AMEAÇAM A ÁREA DE DISTRIBUIÇÃO DE ANFÍBIOS NA AMAZÔNIA BRASILEIRA Dissertação apresentada ao Programa de Pós-Graduação em Biodiversidade Tropical (PPGBIO) da Universidade Federal do Amapá, como requisito parcial à obtenção do título de Mestre em Biodiversidade Tropical. Orientador: Dra. Fernanda Michalski Co-Orientador: Dr. Rafael Loyola MACAPÁ, AP 2017 YURI BRENO DA SILVA E SILVA COMO A EXPANSÃO DE HIDRELÉTRICAS, PERDA FLORESTAL E MUDANÇAS CLIMÁTICAS AMEAÇAM A ÁREA DE DISTRIBUIÇÃO DE ANFÍBIOS NA AMAZÔNIA BRASILEIRA _________________________________________ Dra. Fernanda Michalski Universidade Federal do Amapá (UNIFAP) _________________________________________ Dr. Rafael Loyola Universidade Federal de Goiás (UFG) ____________________________________________ Alexandro Cezar Florentino Universidade Federal do Amapá (UNIFAP) ____________________________________________ Admilson Moreira Torres Instituto de Pesquisas Científicas e Tecnológicas do Estado do Amapá (IEPA) Aprovada em de de , Macapá, AP, Brasil À minha família, meus amigos, meu amor e ao meu pequeno Sebastião. AGRADECIMENTOS Agradeço a CAPES pela conceção de uma bolsa durante os dois anos de mestrado, ao Programa de Pós-Graduação em Biodiversidade Tropical (PPGBio) pelo apoio logístico durante a pesquisa realizada. Obrigado aos professores do PPGBio por todo o conhecimento compartilhado. Agradeço aos Doutores, membros da banca avaliadora, pelas críticas e contribuições construtivas ao trabalho. -

AMPHIBIANS of the RIOZINHO Do ANFRÍSIO Extractive Reserve,Pará
WEB VERSION PARÁ STATE, Eastern Amazon, BRAZIL AMPHIBIANS of the RIOZINHO do ANFRÍSIO Extractive Reserve, Pará 1 Flávio Bezerra Barros1,2; Luís Vicente2 and Henrique M. Pereira2 1Universidade Federal do Pará, Brazil; 2Centro de Biologia Ambiental, Faculdade de Ciências da Universidade de Lisboa, Portugal Photos by Flávio Bezerra Barros, except where indicated. Produced by Tyana Wachter, J. Philipp, & R. Foster, with support from Ellen Hyndman Fund & Andrew Mellon Foundation. © Flávio Bezerra Barros [[email protected]] Universidade Federal do Pará, Brazil; Faculdade de Ciências da Universidade de Lisboa, Portugal © Environmental & Conservation Programs, The Field Museum, Chicago, IL 60605 USA. [[email protected]] [www.fmnh.org/animalguides/] Rapid Color Guide # 271 version 1 08/2010 1 Allophryne ruthveni 2 Allobates femoralis 3 Allobates sp. 4 Dendrophryniscus bokermanni ALLOPHRYNIDAE AROMOBATIDAE AROMOBATIDAE BUFONIDAE (carrying tadpoles) 5 Rhaebo guttatus 6 Rhinella castaneotica 7 Rhinella magnussoni 8 Rhinella gr. margaritifera BUFONIDAE BUFONIDAE BUFONIDAE BUFONIDAE 9 Rhinella gr. margaritifera 10 Rhinella marina 11 Rhinella marina 12 Ceratophrys cornuta BUFONIDAE BUFONIDAE BUFONIDAE CERATOPHRYIDAE (pair in amplexus) (pair in amplexus) 13 Proceratophrys concavitympanum 14 Adelphobates castaneoticus 15 Dendropsophus brevifrons 16 Dendropsophus leucophyllatus CYCLORAMPHIDAE DENDROBATIDAE HYLIDAE HYLIDAE photo: Pedro L.V. Peloso 17 Dendropsophus leucophyllatus 18 Dendropsophus leucophyllatus 19 Dendropsophus melanargyreus 20 Dendropsophus minusculus HYLIDAE HYLIDAE HYLIDAE HYLIDAE (yellow pattern) (giraffe pattern) WEB VERSION PARÁ STATE, Eastern Amazon, BRAZIL AMPHIBIANS of the RIOZINHO do ANFRÍSIO Extractive Reserve, Pará 2 Flávio Bezerra Barros1,2; Luís Vicente2 and Henrique M. Pereira2 1Universidade Federal do Pará, Brazil; 2Centro de Biologia Ambiental, Faculdade de Ciências da Universidade de Lisboa, Portugal Photos by Flávio Bezerra Barros, except where indicated.