First Year Undergraduate Laboratory Course
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chem 353 Derivative Tables
ALCOHOLS Derivatives Mp/ oC Compound Bp /oC 3,5-Dinitro- Phenylurethane Naphthyl- (Mp / oC) benzoate urethane 4-Methylbenzyl alcohol --- (60) 118 79 --- Diphenylmethanol --- (69) 141 136 --- Ethyl p-hydroxybenzoate --- (116) * * * Methyl p-hydroxybenzoate --- (130) * * * Ethanol 78 93 52 79 2-Propanol 83 122 88 106 1-Propanol 97 74 51 80 2-Butanol 99 75 65 97 2-Methyl-1-propanol 108 86 86 104 3-Methyl-2-butanol 113 76 68 110 1-Butanol 116 64 63 71 3-Pentanol 116 101 48 95 2-Pentanol 119 61 --- (oil) 76 2-Chloroethanol 129 92 51 101 2-Methyl-1-butanol 129 70 31 82 3-Methyl-1-butanol 132 61 55 68 4-Methyl-2-pentanol 132 65 143 88 3-Methyl-2-pentanol 134 43 --- 72 1-Pentanol 138 46 46 68 2-Methyl-1-pentanol 148 51 --- 75 2-Ethyl-1-butanol 149 52 --- --- 1-Hexanol 156 58 42 59 Cyclohexanol 161 112 82 129 2-Octanol 179 32 114 63 1-Phenylethanol 203 95 94 106 Benzyl alcohol 205 112 78 134 2-Phenylethanol 219 108 79 119 Ethyl o-hydroxybenzoate 234 * * * Cinnamyl alcohol 257 (33) 121 90 114 4-Methoxybenzyl alcohol 260 (25) --- 92 --- * These compounds are also esters, see that table for derivatives. --- No data available. ESTERS Derivative Mp/ oC Compound Bp /oC Carboxylic (Mp / oC) Acid Methyl p-methylbenzoate 223 (30) 177 Methyl cinnamate 261 (35) 133 Methyl 4-methoxybenzoate 245 (49) 184 Ethyl p-nitrobenzoate --- (57) 241 Methyl p-nitrobenzoate --- (95) 241 Ethyl p-hydroxybenzoate --- (116) 213 Methyl p-hydroxybenzoate --- (130) 213 Methyl benzoate 198 121 Methyl o-toluate 213 102 Ethyl benzoate 213 121 Diethyl succinate 216 190 Methyl phenylacetate 218 76 Methyl salicylate 224 157 Methyl o-chlorobenzoate 230 140 Ethyl salicylate 234 157 Ethyl p-toluate 241 177 Isopropyl salicylate 255 157 Ethyl o-chlorobenzoate 255 140 Dimethyl suberate 268 141 Ethyl 4-methoxybenzoate 270 184 Ethyl cinnamate 271 133 Diethyl phthalate 296 230 --- No data available. -

Ch3ch2cch(Ch3)2 O Ch2ch2cho Ch3cch2ch2ch2cch2ch3
Chem 226 — Problem Set #9 — “Fundamentals of Organic Chemistry,” 4th edition, John McMurry. Chapter 9 2. Name the following aldehydes and ketones. O (a) (b) CH2CH2CHO CH3CH2CCH(CH3)2 H CH3 (c) O O (d) H C O CH3CCH2CH2CH2CCH2CH3 H (a) 2-methyl-3-pentanone, (b) 3-phenylpropanal, (c) 2,6-octanedione, (d) (1R,2R)-2-methylcyclohexanecarbaldehyde 4. How could you prepare pentanal from the following starting materials? (a) 1-pentanol, (b) CH3CH2CH2CH2COOH, (c) 5-decene PCC (a) CH3CH2CH2CH2CH2OH CH3CH2CH2CH2CHO CH2Cl2 O 1. LiAlH4 CH3CH2CH2CH2C OH CH3CH2CH2CH2CH2OH H O+ (b) 2. 3 PCC CH3CH2CH2CH2CH2OH CH3CH2CH2CH2CHO CH2Cl2 KMnO4 CH3CH2CH2CH2CH CHCH2CH2CH2CH3 + H3O O LiAlH (c) 1. 4 CH3CH2CH2CH2C OH + CH3CH2CH2CH2CH2OH 2. H3O PCC CH3CH2CH2CH2CH2OH CH3CH2CH2CH2CHO CH2Cl2 5. How could you prepare 2-hexanone from the following starting materials? (a) 2-hexanol, (b) 1-hexyne, (c) 2-methyl-1-hexene OH O K2CrO7 (a) CH3CH2CH2CH2CHCH3 CH3CH2CH2CH2CCH3 O H2SO4 (b) CH3CH2CH2CH2C CH CH3CH2CH2CH2CCH3 HgSO4 H2O CH3 O KMnO4 (c) CH3CH2CH2CH2C CH2 CH3CH2CH2CH2CCH3 + H3O (b) The enol that initially forms here undergoes keto-enol tautomerism. Terminal alkynes give methyl ketones; they follow Markovnikov’s rule. (c) Potassium permanganate under basic conditions would hydroxylate the alkene to form a vicinal diol, but under acidic conditions it completely cleaves the double bond. The =CH2 group would become carbon dioxide. 6. How would you carry out the following transformations? More than one step may be required. (a) 3-hexene 3-hexanone (b) benzene 1-phenylethanol H OH H2O (a) CH3CH2CH CHCH2CH3 CH3CH2CH CHCH2CH3 H SO 2 4 H O K2Cr2O7 CH3CH2CH CCH2CH3 O CH3CCl (b) C CH3 AlCl3 O H NaBH4 C CH3 OH 9. -

Tin-Free Alternatives to the Barton-Mccombie Deoxygenation of Alcohols to Alkanes Involving Reductive Electron Transfer
The French connecTion CHIMIA 2016, 70, No. 1/2 67 doi:10.2533/chimia.2016.67 Chimia 70 (2016) 67–76 © Swiss Chemical Society Tin-free Alternatives to the Barton- McCombie Deoxygenation of Alcohols to Alkanes Involving Reductive Electron Transfer Ludwig Chenneberg and Cyril Ollivier* Abstract: Echoing the recent celebration of the fortieth anniversary of the Barton-McCombie reaction, this review aims to explore another facet of radical processes for deoxygenation of alcohols by considering SET (single electron transfer) reduction of carboxylic ester, thiocarbonate and thiocarbamate derivatives. Various protocols have been developed relying on the use of organic and organometallic SET reagents, electrochemi- cal conditions, photoinduced electron transfer processes and visible-light photoredox catalysis. Applications to the synthesis of molecules of interest provide a glimpse into the scope of these different approaches. Keywords: Alcohols · Deoxygenation · Electron transfer · Radicals · Reduction 1. Introduction phorus derivatives and less toxic metals.[1] secondary and tertiary alcohols.[7] In 2015, Interesting perspectives have opened up the radical community celebrated the 40th Radical chemistry has witnessed an ex- with the use of organoboranes[4] and com- anniversary of the discovery of the Barton- plosive growth over the last three decades. pounds responsible for electron-transfer McCombie deoxygenation reaction, unan- Owing to their mildness and high com- reactions have been increasingly seen as imously considered as the most common -

Ep 3048090 A1
(19) TZZ¥ZZZ_T (11) EP 3 048 090 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 27.07.2016 Bulletin 2016/30 C07C 29/141 (2006.01) C07C 29/151 (2006.01) C07C 29/153 (2006.01) (21) Application number: 14845165.1 (86) International application number: (22) Date of filing: 17.09.2014 PCT/KR2014/008666 (87) International publication number: WO 2015/041471 (26.03.2015 Gazette 2015/12) (84) Designated Contracting States: •KIM,Jin Soo AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Daejeon 305-738 (KR) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • CHOE, Yong Jin PL PT RO RS SE SI SK SM TR Daejeon 305-738 (KR) Designated Extension States: • CHOI, Sun Hyuk BA ME Daejeon 305-738 (KR) (30) Priority: 17.09.2013 KR 20130111558 (74) Representative: Goddar, Heinz J. Boehmert & Boehmert (71) Applicant: LG Chem, Ltd. Anwaltspartnerschaft mbB Seoul 150-721 (KR) Patentanwälte Rechtsanwälte Pettenkoferstrasse 20-22 (72) Inventors: 80336 München (DE) • NAM, Hyun Daejeon 305-738 (KR) (54) METHOD FOR PREPARING ALKANOL (57) Provided are a method of preparing an alkanol and a device for preparing the same. According to the method and device, economic feasibility and stability of a preparation process may be enhanced, and mass production of an alkanol may be performed. EP 3 048 090 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 048 090 A1 Description [Technical Field] 5 [0001] The present application relates to a method of preparing an alkanol and a device for preparing the same. -

Chem 31 Lab Google
SANTA CLARA UNIVERSITY SUMMER TERM 2019 ORGANIC CHEMISTRY I CHEMISTRY 31L LABORATORY SYLLABUS INSTRUCTORS Raymond, Gipson, Ph.D.; Beatrice Ruhland, Ph.D. TIMES • Monday/Wednesday: 8:00-12:30 p.m. • Monday/Wednesday: 4:30-9:00 p.m. • Tuesday/Thursday: 8:00-12:30 p.m. • Tuesday/Thursday: 4:30-9:00 p.m. Laboratories begin on Monday, June 17th and are held in DS 126 MATERIALS • Laboratory syllabus: available on Chemistry 31-33 laboratory site (https://sites.google.com/a/scu.edu/organic-chemistry-laboratory/), or for purchase from Copy Craft Printing (341 Lafayette St.), • Laboratory Techniques in Organic Chemistry by Mohrig, Alberg, Hofmeister, Schatz and Hammond, 4th Edition • Bound laboratory notebook embossed with "Santa Clara University", Scientific Notebook Company: available from the bookstore • Safety splash goggles • Laboratory coat: available from the bookstore LABORATORY GUIDELINES • The laboratories in Daly Science 100 were modernized and redesigned in 1994 with safety as the primary concern. The main improvement in these labs was increasing the number of hoods so that each student has a workstation in a hood, two students per hood. This significant enhancement in safety protects everyone because many organic compounds are volatile and using a hood minimizes your exposure to fumes. Therefore, virtually 100% of your chemical work should be performed in a fume hood. • To do organic chemistry safely, you should treat all chemicals with respect; gloves should be worn unless otherwise directed by the instructor. • Care of the organic laboratory is important because some 200 students share this organic facility each term, so we must regularly maintain this facility. -

CE-123 on Mesocorticolimbic Dopamine System
biomolecules Article Neurophysiological and Neurochemical Effects of the Putative Cognitive Enhancer (S)-CE-123 on Mesocorticolimbic Dopamine System 1, 1, 2 2 Claudia Sagheddu y , Nicholas Pintori y, Predrag Kalaba , Vladimir Dragaˇcevi´c , Gessica Piras 1, Jana Lubec 3, Nicola Simola 1, Maria Antonietta De Luca 1 , Gert Lubec 3,* and Marco Pistis 1,4,* 1 Department of Biomedical Sciences, Division of Neuroscience and Clinical Pharmacology, University of Cagliari, 09042 Monserrato, Italy; [email protected] (C.S.); [email protected] (N.P.); [email protected] (G.P.); [email protected] (N.S.); [email protected] (M.A.D.L.) 2 Department of Pharmaceutical Chemistry, Faculty of Life Sciences, University of Vienna, 1010 Vienna, Austria; [email protected] (P.K.); [email protected] (V.D.) 3 Department of Neuroproteomics, Paracelsus Medical University, 5020 Salzburg, Austria; [email protected] 4 Neuroscience Institute, National Research Council of Italy (CNR), Section of Cagliari, 09100 Cagliari, Italy * Correspondence: [email protected] (G.L.); [email protected] (M.P.); Tel.: +43-(0)-6622420-0 (G.L.); +39-070-675-4324 (M.P.) These authors contributed equally to this work. y Received: 2 April 2020; Accepted: 15 May 2020; Published: 18 May 2020 Abstract: Treatments for cognitive impairments associated with neuropsychiatric disorders, such as attention deficit hyperactivity disorder or narcolepsy, aim at modulating extracellular dopamine levels in the brain. CE-123 (5-((benzhydrylsulfinyl)methyl) thiazole) is a novel modafinil analog with improved specificity and efficacy for dopamine transporter inhibition that improves cognitive and motivational processes in experimental animals. We studied the neuropharmacological and behavioral effects of the S-enantiomer of CE-123 ((S)-CE-123) and R-modafinil in cognitive- and reward-related brain areas of adult male rats. -

The Reaction Kinetics of Neutral Free Radicals and Radical Ions Studied by Laser Flash Photolysis
The Reaction Kinetics of Neutral Free Radicals and Radical Ions Studied by Laser Flash Photolysis Robert A. Friedline Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Chemistry James M. Tanko, Chair Dr. Alan Esker Dr. Timothy Long Dr. James Mahaney Dr. J. Paige Stevenson April 21st, 2004 Blacksburg, Virginia Keywords: Free radical, alkoxy, t-butoxyl, radical anion, laser flash photolysis Copyright 2004, Robert A. Friedline The Reaction Kinetics of Neutral Free Radicals and Radical Ions Studied by Laser Flash Photolysis by Robert A. Friedline James M. Tanko, Chair Chemistry (Abstract) t-Butoxyl radical has been used as a chemical model for hydrogen abstractions in many enzymatic and biological systems. However, the question has arisen as to how well this reactive intermediate mimics these systems. In addressing this concern, absolute rate constants and Arrhenius parameters for hydrogen abstraction by t-butoxyl radical were measured for a broad class of substrates including amines, hydrocarbons, and alcohols using laser flash photolysis. Initially, no obvious reactivity relationship between rate constant and substrate structure was observed for these homolytic reactions. However, by closely examining the Arrhenius parameters for hydrogen abstraction, a pattern was revealed. For substrates with C-H bond dissociation energy (BDE) > 92 kcal/mole, activation energy increases with increasing BDE (as expected). However, for substrates with a lower BDE, the activation energy levels out at approximately 2 kcal/mole, essentially independent of structure. Viscosity studies with various solvents were conducted, ruling out the possibility of diffusion-controlled reactions. -

Ep 0242801 B1
~" ' MM II II II II II Ml II I Ml II II I II J European Patent Office r\ r%r\+ n » © Publication number: 0 242 801 B1 Office europeen- desj brevets^ . EUROPEAN PATENT SPECIFICATION © Date of publication of patent specification: 10.03.93 © Int. CI.5: C07F 3/02 © Application number: 87105638.8 @ Date of filing: 16.04.87 © Hydrocarbyloxy magnesium halides. ® Priority: 18.04.86 US 853496 © Proprietor: LITHIUM CORPORATION OF AMER- ICA @ Date of publication of application: 449 North Cox Road 28.10.87 Bulletin 87/44 Gastonia NC 28054(US) © Publication of the grant of the patent: @ Inventor: Mehta, Vijay Chandrakant 10.03.93 Bulletin 93/10 3100 Imperial Drive Gastonia North Carolina 28054(US) © Designated Contracting States: AT BE CH DE ES FR GB GR IT LI LU NL SE © Representative: Zumstein, Fritz, Dr. et al © References cited: Dr. F. Zumstein Dr. E. Assmann Dipl.-lng. F. DE-A- 2 724 971 Klingseisen Brauhausstrasse 4 DE-A- 2 743 415 W-8000 Munchen 2 (DE) JOURNAL OF AMERICAN CHEMICAL SOCI- ETY vol. 54, June 1932, pages 2453, 2455; D L YABROFF et al.: "The preparation and prop- erties of tertiary butyl phenylacetate"*page 2454, first paragraph* JOURNAL OF ORGANOMETALLIC CHEMIS- TRY VOL. 42, 1972, pages 9-17; Y TUROVA et al.: "Alkoxymagnesium Halides" * page 12, 00 last 2 paragraphs IDEM 00 CM CM Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -
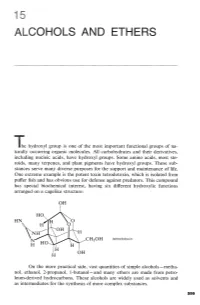
Alcohols and Ethers
ALCOHOLS AND ETHERS The hydroxyl group is one of the most important functional groups of na- turally occurring organic molecules. All carbohydrates and their derivatives, including nucleic acids, have hydroxyl groups. Some amino acids, most ste- roids, many terpenes, and plant pigments have hydroxyl groups. These sub- stances serve many diverse purposes for the support and maintenance of life. One extreme example is the potent toxin tetrodotoxin, which is isolated from puffer fish and has obvious use for defense against predators. This compound has special biochemical interest, having six different hydroxylic functions arranged on a cagelike structure: tetrodotoxin On the more practical side, vast quantities of simple alcohols - metha- nol, ethanol, 2-propanol, 1-butan01 - and many ethers are made from petro- leum-derived hydrocarbons. These alcohols are widely used as solvents and as intermediates for the synthesis of more complex substances. 15 Alcohols and Ethers The reactions involving the hydrogens of alcoholic OH groups are expected to be similar to those of water, HOH, the simplest hydroxylic com- pound. Alcohols, ROH, can be regarded in this respect as substitution products of water. However, with alcohols we shall be interested not only in reactions that proceed at the 8-H bond but also with processes that result in cleavage of the C-0 bond, or changes in the organic group R. The simple ethers, ROR, do not have 0-H bonds, and most of their reactions are limited to the substituent groups. The chemistry of ethers, there- fore, is less varied than that of alcohols. This fact is turned to advantage in the widespread use of ethers as solvents for a variety of organic reactions, as we already have seen for Grignard reagents (Section 14- 10). -

Applying the Principles of Green Chemistry to Selected Traditional Organic Chemistry Reactions
Applying the Principles of Green Chemistry to Selected Traditional Organic Chemistry Reactions By © Steven White A thesis submitted in partial fulfillment of the requirements for the degree of Bachelor of Science, Honours Environmental Science (Chemistry Stream) Grenfell Campus Memorial University of Newfoundland Corner Brook, NL ii ACKNOWLEDGEMENTS I thank Dr. Sudhir Abhyankar for his help throughout the research project and Dr. Parkinson for his help with the infrared spectrometer. Thanks to all of the lab technicians who helped me along the way. You opened many doors for me. Also, a thank you for the Environmental Science Study Grant which was used to purchase various chemicals for different reactions. iii ABSTRACT The primary objective of this project was to apply the principles of green chemistry to selected traditional synthetic organic chemistry reactions. The reactions studied in the lab were: amide formation, oxidation of alcohols with KMnO4 and LiCl/H2O2, oxidation of alkylarenes with KMnO4, reduction of β-ketoesters with yeast, and formation of esters by phase transfer catalysis. Microwave irradiation, biocatalysis, phase transfer catalysis, solvent-free conditions, and/or alternate solvents were used to accomplish these syntheses. Results were analyzed by the traditionally used percent yield calculations and selected green chemistry metrics. The main limitation of the results was that most products were not completely purified due to time constraints. For amidification it was found that results obtained using a commercial oven were better than results obtained using a domestic oven. Oxidation with KMnO4 gave higher yields at one week reaction time under solvent-free conditions than under reflux in dichloromethane for 1.5 h. -

United States Patent (19) 11 Patent Number: 5,041,635 Narisada Et Al
United States Patent (19) 11 Patent Number: 5,041,635 Narisada et al. (45) Date of Patent: "Aug. 20, 1991 (54) 5(2)-7-(ENDO-3-BENZENESUL Jul. 17, 1986 (JP) Japan .............................. 61-1692.57 FONAMIDOBICYCLO(2.2.1]HEPT-EXO-2- 51) Int. Cl. ............................................ C07C311/20 YL)-5-HEPTENOIC ACID, SODIUM SALT 52 U.S. C. .................................................... 562/427 75) Inventors: Masayuki Narisada, Osaka; Mitsuaki 58) Field of Search ......................................... 562/427 Ohtani, Nara, both of Japan 73) Assignee: Shionogi & Co., Ltd., Osaka, Japan (56) References Cited U.S. PATENT DOCUMENTS *) Notice: The portion of the term of this patent subsequent to Aug. 29, 2006 has been 4,861,913 8/1989 Narisada et al. ...................... 549/23 disclaimed. OTHER PUBLICATIONS (21) Appl. No.: 541,491 Irwin, et al., J.A.C.S., pp. 8476-84.82 (1976), 98. 22 Filed: Jun. 20, 1990 Narisada et al., J. Med, Chem., 31(1988), pp. 1847-1854. Primary Examiner-Joseph P. Brust Related U.S. Application Data Attorney, Agent, or Firn-Wenderoth, Lind & Ponack 60 Continuation-in-part of Ser. No. 327,778, Mar. 23, 1989, which is a division of Ser. No. 927,823, Nov. 5, 57 ABSTRACT 1986, Pat. No. 4,861,913. There is provided 5(Z)-7-(endo-3-benzenesul (30) Foreign Application Priority Data fonamidobicyclo[2.2.1]hept-exo-2-yl)-5-heptenoic acid, Nov. 18, 1985 (JP) Japan ................................ 60-25915.4 sodium salt which is useful as an antithrombotic, anti Feb, l3, 1986 JP Japan .................................. 61-301.30 vasoconstricting and anti-bronchoconstricting agent. May 19, 1986 JP Japan ............................... -

Combined Prohibited and Restricted Chemical Lists
Dedicated to protecting and improving the health and environment of the people of Colorado Combined Prohibited and Restricted Chemical Lists This document is a combined and alphabetized list of the Prohibited and Restricted Chemicals as defined and listed in the Rules and Regulations Governing Schools in the State of Colorado, 6 CCR 1010-6, Appendices A, B, and B2. This combined list can be used as a supplement to the Rules and Regulations and as a quick reference format when conducting such activities as a chemical inventory or during the chemical procurement process. Shelf life descriptions are provided for each chemical to assist in evaluating a timeframe for safe chemical use and storage. In general, chemical shelf life descriptions are as follows: POOR - less than 1 year; FAIR - 1 to 3 years; GOOD - 3 to 5 years; and EXCELLENT or INDEFINITE - greater than 5 years. Additional oversight for chemicals with a shelf life of less than 3 years is important to ensure that they are not stored or used past their shelf life or expiration date, at which point they may become unstable and dangerous. Completion of Table 2 on the Restricted Chemical Variance Application Form is required for any chemicals with a shelf life of less than 3 years and/or those chemicals that are known to have issues with container integrity or other hazards associated with their use and storage in a school environment. Please see "Limited Shelf Life – Table 2 Required?" column on this list before completing the variance application form. Please note that the hazard information and shelf life descriptions provided for the listed chemicals is not intended to address all safety concerns.