Relationshipbetweenupophilicity
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Exam 1 (February 23, 2004) ID# ______
Chemistry 211 Name ___________________________ Exam 1 (February 23, 2004) ID# ___________________________ 10 1. You desire to synthesize 3-ethyl-3-pentanol starting with an ester. (i) What would be the name of the ester, and what is the name for the Grignard reagent (e.g., methyl magnesium bromide)? (ii) For the carbons shown in the product, show plausible hydrocarbons that you could start with to produce the ester and the Grignard reagent (as in a retrosynthesis). 12 2. (i) Show the step-by-step process required to produce propyllithium, which requires a free radical reaction mechanism, . (ii) Show the complete reaction mechanism for reaction between propyllithium and the correct ketone to produce 3-propyl-3-pentanol. (iii) Propose a possible reaction mechanism by which dipropyl cuprate (Cu+ with two propyl groups attached) could react with ethyl bromide to produce a new hydrocarbon. (This is a thinking exercise! So, think! () 8 3. As mentioned in the text, diethyl ether, pentane, and 1-butanol have similar molar masses, but different physical properties. Boiling points are 35oC, 36oC, and 117oC, respectively. Their respective solubilities in water are 7.5g/100mL, insoluble, and 9g/100mL. (i) Draw structures for each of these compounds. (ii) Justify the observed boiling points and their solubilities. 16 4. Draw structures of the following compounds 2,3-heptanediol isopropyllithium benzylmagnesium bromide benzoic acid benzaldehylde dimethyl sulfide t-butyl methanoate dibutyl ketone 12 5. Alcohols can be oxidized to produce other compounds, and can be produced by reduction. For the reactions shown below, show the structure for the expected product (if reaction does not occur, state: No Reaction) when treated with the indicated oxidizing or reducing agents. -

Grignard Reaction: Synthesis of Triphenylmethanol
*NOTE: Grignard reactions are very moisture sensitive, so all the glassware in the reaction (excluding the work-up) should be dried in an oven with a temperature of > 100oC overnight. The following items require oven drying. They should be placed in a 150mL beaker, all labeled with a permanent marker. 1. 5mL conical vial (AKA: Distillation receiver). 2. Magnetic spin vane. 3. Claisen head. 4. Three Pasteur pipettes. 5. Two 1-dram vials (Caps EXCLUDED). 6. One 2-dram vial (Caps EXCLUDED). 7. Glass stirring rod 8. Adaptor (19/22.14/20) Grignard Reaction: Synthesis of Triphenylmethanol Pre-Lab: In the “equations” section, besides the main equations, also: 1) draw the equation for the production of the byproduct, Biphenyl. 2) what other byproduct might occur in the reaction? Why? In the “observation” section, draw data tables in the corresponding places, each with 2 columns -- one for “prediction” (by answering the following questions) and one for actual drops or observation. 1) How many drops of bromobenzene should you add? 2) How many drops of ether will you add to flask 2? 3) 100 µL is approximately how many drops? 4) What are the four signs of a chemical reaction? (Think back to Chem. 110) 5) How do the signs of a chemical reaction apply to this lab? The Grignard reaction is a useful synthetic procedure for forming new carbon- carbon bonds. This organometallic chemical reaction involves alkyl- or aryl-magnesium halides, known as Grignard 1 reagents. Grignard reagents are formed via the action of an alkyl or aryl halide on magnesium metal. -

Organic Chemistry Laboratory II Preparation of Triphenylmethanol (Grignard Reaction) Experiment Procedure (Printable Pdf Format)
grigdes http://www.organicchem.org/oc2web/lab/exp/grig/grigdesbenzophenone... Organic Chemistry II | Lecture | Laboratory Organic Chemistry Laboratory II Preparation of Triphenylmethanol (Grignard Reaction) Experiment Procedure (Printable pdf format) Introduction In this two-week experiment, triphenylmethanol will be synthesized through a Grignard reaction. Students will work individually to prepare the Grignard reagent by reacting bromobenzene with solid magnesium in diethylether. The prepared Grignard reagent, phenylmagnesium bromide, will then be combined with bezophenone to form the desired triphenylmethanol product. Procedure NOTE: All glassware must be extremely dry! Place glassware in the drying oven for at least 10 minutes before beginning the reaction. Preparation of the Grignard Reagent Add ~0.5g of magnesium turnings and a magnetic stir bar to a dry 100 ml round-bottomed flask, and place the flask in the drying oven for 30 minutes. Remove the flask from the oven, clamp it to a ring stand, insert a reflux condenser, fit the flask into a heating mantle and immediately attach a drying tube to the top of the reflux condenser. Position the flask in the heating mantle on a stirrer/hotplate. Set the reaction up on the side of the hood near to the faucet labeled for distillation. Allow the reaction flask to cool to room temperature completely before proceeding. While the flask is cooling, place a 150ml beaker into the drying oven. While the flask is cooling, add 3.5 g of dry bromobenzene to a dry 50 mL Erlenmeyer flask and dissolve it in 5.0 mL of anhydrous diethyl ether. Use a glass powder funnel to dispense the bromobenze and ether to the Erlenmyer flask. -

Chem 353 Derivative Tables
ALCOHOLS Derivatives Mp/ oC Compound Bp /oC 3,5-Dinitro- Phenylurethane Naphthyl- (Mp / oC) benzoate urethane 4-Methylbenzyl alcohol --- (60) 118 79 --- Diphenylmethanol --- (69) 141 136 --- Ethyl p-hydroxybenzoate --- (116) * * * Methyl p-hydroxybenzoate --- (130) * * * Ethanol 78 93 52 79 2-Propanol 83 122 88 106 1-Propanol 97 74 51 80 2-Butanol 99 75 65 97 2-Methyl-1-propanol 108 86 86 104 3-Methyl-2-butanol 113 76 68 110 1-Butanol 116 64 63 71 3-Pentanol 116 101 48 95 2-Pentanol 119 61 --- (oil) 76 2-Chloroethanol 129 92 51 101 2-Methyl-1-butanol 129 70 31 82 3-Methyl-1-butanol 132 61 55 68 4-Methyl-2-pentanol 132 65 143 88 3-Methyl-2-pentanol 134 43 --- 72 1-Pentanol 138 46 46 68 2-Methyl-1-pentanol 148 51 --- 75 2-Ethyl-1-butanol 149 52 --- --- 1-Hexanol 156 58 42 59 Cyclohexanol 161 112 82 129 2-Octanol 179 32 114 63 1-Phenylethanol 203 95 94 106 Benzyl alcohol 205 112 78 134 2-Phenylethanol 219 108 79 119 Ethyl o-hydroxybenzoate 234 * * * Cinnamyl alcohol 257 (33) 121 90 114 4-Methoxybenzyl alcohol 260 (25) --- 92 --- * These compounds are also esters, see that table for derivatives. --- No data available. ESTERS Derivative Mp/ oC Compound Bp /oC Carboxylic (Mp / oC) Acid Methyl p-methylbenzoate 223 (30) 177 Methyl cinnamate 261 (35) 133 Methyl 4-methoxybenzoate 245 (49) 184 Ethyl p-nitrobenzoate --- (57) 241 Methyl p-nitrobenzoate --- (95) 241 Ethyl p-hydroxybenzoate --- (116) 213 Methyl p-hydroxybenzoate --- (130) 213 Methyl benzoate 198 121 Methyl o-toluate 213 102 Ethyl benzoate 213 121 Diethyl succinate 216 190 Methyl phenylacetate 218 76 Methyl salicylate 224 157 Methyl o-chlorobenzoate 230 140 Ethyl salicylate 234 157 Ethyl p-toluate 241 177 Isopropyl salicylate 255 157 Ethyl o-chlorobenzoate 255 140 Dimethyl suberate 268 141 Ethyl 4-methoxybenzoate 270 184 Ethyl cinnamate 271 133 Diethyl phthalate 296 230 --- No data available. -

Ch3ch2cch(Ch3)2 O Ch2ch2cho Ch3cch2ch2ch2cch2ch3
Chem 226 — Problem Set #9 — “Fundamentals of Organic Chemistry,” 4th edition, John McMurry. Chapter 9 2. Name the following aldehydes and ketones. O (a) (b) CH2CH2CHO CH3CH2CCH(CH3)2 H CH3 (c) O O (d) H C O CH3CCH2CH2CH2CCH2CH3 H (a) 2-methyl-3-pentanone, (b) 3-phenylpropanal, (c) 2,6-octanedione, (d) (1R,2R)-2-methylcyclohexanecarbaldehyde 4. How could you prepare pentanal from the following starting materials? (a) 1-pentanol, (b) CH3CH2CH2CH2COOH, (c) 5-decene PCC (a) CH3CH2CH2CH2CH2OH CH3CH2CH2CH2CHO CH2Cl2 O 1. LiAlH4 CH3CH2CH2CH2C OH CH3CH2CH2CH2CH2OH H O+ (b) 2. 3 PCC CH3CH2CH2CH2CH2OH CH3CH2CH2CH2CHO CH2Cl2 KMnO4 CH3CH2CH2CH2CH CHCH2CH2CH2CH3 + H3O O LiAlH (c) 1. 4 CH3CH2CH2CH2C OH + CH3CH2CH2CH2CH2OH 2. H3O PCC CH3CH2CH2CH2CH2OH CH3CH2CH2CH2CHO CH2Cl2 5. How could you prepare 2-hexanone from the following starting materials? (a) 2-hexanol, (b) 1-hexyne, (c) 2-methyl-1-hexene OH O K2CrO7 (a) CH3CH2CH2CH2CHCH3 CH3CH2CH2CH2CCH3 O H2SO4 (b) CH3CH2CH2CH2C CH CH3CH2CH2CH2CCH3 HgSO4 H2O CH3 O KMnO4 (c) CH3CH2CH2CH2C CH2 CH3CH2CH2CH2CCH3 + H3O (b) The enol that initially forms here undergoes keto-enol tautomerism. Terminal alkynes give methyl ketones; they follow Markovnikov’s rule. (c) Potassium permanganate under basic conditions would hydroxylate the alkene to form a vicinal diol, but under acidic conditions it completely cleaves the double bond. The =CH2 group would become carbon dioxide. 6. How would you carry out the following transformations? More than one step may be required. (a) 3-hexene 3-hexanone (b) benzene 1-phenylethanol H OH H2O (a) CH3CH2CH CHCH2CH3 CH3CH2CH CHCH2CH3 H SO 2 4 H O K2Cr2O7 CH3CH2CH CCH2CH3 O CH3CCl (b) C CH3 AlCl3 O H NaBH4 C CH3 OH 9. -
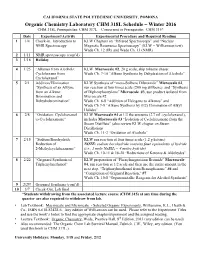
318 Lab Schedule
CALIFORNIA STATE POLYTECHNIC UNIVERSITY, POMONA Organic Chemistry Laboratory CHM 318L Schedule – Winter 2016 CHM 318L Prerequisites: CHM 317L Concurrent or Prerequisite: CHM 315* Date Experiment/Activity Experimental Procedure and Required Reading 1 1/4 Check-in. Introduction to KLW Chapters on “Infrared Spectroscopy” and “Nuclear NMR Spectroscopy Magnetic Resonance Spectroscopy” (KLW = Williamson text) Wade Ch. 12 (IR) and Wade Ch. 13 (NMR) 2 1/11 NMR spectroscopy (cont’d) 3 1/18 Holiday 4 1/25 “Alkenes from Alcohols: KLW Macroscale #2, 20 g scale, skip toluene chaser Cyclohexene from Wade Ch. 7-10 “Alkene Synthesis by Dehydration of Alcohols” Cyclohexanol” 5 2/1 Addition/Elimination KLW Synthesis of “meso-Stilbene Dibromide” Microscale #2, “Synthesis of an Alkyne run reaction at four times scale (200 mg stillbene); and “Synthesis from an Alkene: of Diphenylacetylene” Microscale #3, use product isolated from Bromination and Microscale #2 Dehydrobromination” Wade Ch. 8-8 “Addition of Halogens to Alkenes” and Wade Ch 7-9 “Alkene Synthesis by (E2) Elimination of Alkyl Halides” 6 2/8 “Oxidation: Cyclohexanol KLW Macroscale #4 at 1/3 the amounts (2.7 mL cyclohexanol), to Cyclohexanone” includes Macroscale #3 “Isolation of Cyclohexanone from the Steam Distillate” (also review KLW chapter on Steam Distillation) Wade Ch. 11-2 “Oxidation of Alcohols” 7 2/15 “Sodium Borohydride KLW run reaction at four times scale (1.2 g ketone) Reduction of NOTE: sodium borohydride contains four equivalents of hydride 2-Methylcyclohexanone” (i.e., 1 mole NaBH4 = 4 moles hydride) Wade Ch. 10-11 & 18-20 “Reductions of Ketones & Aldehydes” 8 2/22 “Grignard Synthesis of KLW preparation of “Phenylmagnesium Bromide” Macroscale Triphenylmethanol” #4, run reaction at 1/2 scale and then use the entire amount in the next step, “Triphenylmethanol from Benzophenone” #6 and “Completion of Grignard Reaction,” #7 Wade Ch. -

Revised Group Additivity Values for Enthalpies of Formation (At 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds
Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds Cite as: Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 Submitted: 17 January 1996 . Published Online: 15 October 2009 N. Cohen ARTICLES YOU MAY BE INTERESTED IN Additivity Rules for the Estimation of Molecular Properties. Thermodynamic Properties The Journal of Chemical Physics 29, 546 (1958); https://doi.org/10.1063/1.1744539 Critical Evaluation of Thermochemical Properties of C1–C4 Species: Updated Group- Contributions to Estimate Thermochemical Properties Journal of Physical and Chemical Reference Data 44, 013101 (2015); https:// doi.org/10.1063/1.4902535 Estimation of the Thermodynamic Properties of Hydrocarbons at 298.15 K Journal of Physical and Chemical Reference Data 17, 1637 (1988); https:// doi.org/10.1063/1.555814 Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 25, 1411 © 1996 American Institute of Physics for the National Institute of Standards and Technology. Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds N. Cohen Thermochemical Kinetics Research, 6507 SE 31st Avenue, Portland, Oregon 97202-8627 Received January 17, 1996; revised manuscript received September 4, 1996 A program has been undertaken for the evaluation and revision of group additivity values (GAVs) necessary for predicting, by means of Benson's group additivity method, thermochemical properties of organic molecules. This review reports on the portion of that program dealing with GAVs for enthalpies of formation at 298.15 K (hereinafter abbreviated as 298 K) for carbon-hydrogen and carbon-hydrogen-oxygen compounds. -

Grignard Synthesis of Triphenylmethanol Reactions That Form Carbon-Carbon Bonds Are Among the Most Useful to the Synthetic Organic Chemist
1 Experiment 12: Grignard Synthesis of Triphenylmethanol Reactions that form carbon-carbon bonds are among the most useful to the synthetic organic chemist. In 1912, Victor Grignard received the Nobel prize in chemistry for his discovery of a new series of reactions that result in the formation of a carbon-carbon bond. A Grignard synthesis first involves the preparation of an organomagnesium reagent via the reaction of an alkyl bromide with magnesium metal: δ– δ+ R Br + Mg R MgBr The resulting “Grignard reagent” acts as both a good nucleophile and a strong base. Its nucleophilic character allows it to react with the electrophilic carbon in a carbonyl group, thus forming the carbon-carbon bond. Its basic property means that it will react with acidic compounds, such as carboxylic acids, phenols, thiols and even alcohols and water; therefore, reaction conditions must be free from acids and strictly anhydrous. Grignard reagents will also react with oxygen to form hydroperoxides, thus they are highly unstable when exposed to the atmosphere and are generally not isolated from solution. For a variety of reasons, anhydrous diethyl ether is the solvent of choice for carrying out a Grignard synthesis. Vapors from the highly volatile solvent help to prevent oxygen from reaching the reaction solution. In addition, evidence suggests that the ether molecules actually coordinate with and help stabilize the Grignard reagent: Et Et O R Mg Br O Et Et The magnesium metal used in the synthesis contains a layer of oxide on the surface that prevents it from reacting with the alkyl bromide. The pieces of metal must be gently scratched while in the ether solution to expose fresh surface area so that the reaction can commence. -

The Effects of Stoichiometry and Starting Material on the Product Identity and Yield in Grignard Addition Reactions
Supplementary information for Comprehensive Organic Chemistry Experiments for the Laboratory Classroom © The Royal Society of Chemistry 2017 The effects of stoichiometry and starting material on the product identity and yield in Grignard addition reactions Supplementary Material This experiment has been performed both in the 150 person standard introductory organic chemistry laboratory (taught primarily by undergraduate teaching assistants in five sections of 30-40 students) and in a special introductory organic chemistry laboratory for freshman, taught to 23 students in sections of 7 and 16. This course is the first organic laboratory for these students but has been taught in the spring semester along with the second semester of organic lecture. The lab periods for this course are five hours long, and this experiment is typically performed in the latter half of the semester. This experiment is used to illustrate to students the importance of planning their time in lab; they must be out of the lab in the 5 hours allotted. While many students finish the experiment in this time, others plan to finish the following week along with a shorter experiment. Those who were running behind were encouraged to finish through the drying of their organic layer with MgSO4 and set up the distillation the following week. When doing this they should make sure that their organic layer is in a closed container to prevent evaporation of their product. Moisture Sensitive Conditions There are many diverse protocols for maintaining the anhydrous conditions required to successfully prepare and utilize Grignard reagents. We typically open a fresh can of anhydrous ether and dispense it directly, without additional drying in a still or air sensitive techniques for solvent transfers. -

CHM205 Chemicals by Experiment Tuesday, November 17, 2015 3:14:15 PM Experiment Title Chemical Name Concentration Acetaminophen Synthesis Acetic Anhydride Liquid
CHM205 Chemicals by Experiment Tuesday, November 17, 2015 3:14:15 PM Experiment Title Chemical Name Concentration Acetaminophen Synthesis Acetic anhydride liquid Acetaminophen Synthesis p-aminophenol solid Alcohols to Alkyl chlorides 2-pentanol liquid Alcohols to Alkyl chlorides Hydrochloric acid 12 M Alcohols to Alkyl chlorides Sodium carbonate solid Alcohols to Alkyl chlorides Hydrobromic acid 48% w/v Alcohols to Alkyl chlorides Sodium sulfate anhydrous solid Alcohols to Alkyl chlorides sec-phenethyl alcohol liquid Alcohols to Alkyl chlorides Benzyl alcohol liquid Alcohols to Alkyl chlorides t-butanol liquid Alcohols to Alkyl chlorides 1-pentanol liquid Alcohols to Alkyl chlorides Sodium carbonate 10% w/v Diels Alder Reaction 2,3-dimethyl-1,3-butadiene liquid Diels Alder Reaction Maleic anhydride solid Diels Alder Reaction Ethanol 95% Liquid Diels Alder Reaction Hexane liquid Diels Alder Reaction Cyclohexane liquid Diels Alder Reaction Calcium chloride solid Esterification methanol liquid Esterification Sodium carbonate 10% w/v Esterification 1-propanol liquid Esterification 1-butanol liquid Esterification trans-cinnamic acid solid Esterification Isoamyl alcohol liquid Esterification Isopropyl alcohol liquid Esterification Benzyl alcohol liquid Esterification Sulfuric acid conc. 18 M Esterification 1-pentanol liquid Esterification Isobutyl alcohol liquid Esterification Ethanol 95% liquid Page 1 of 3 Experiment Title Chemical Name Concentration Extraction of Beta Carotene Cyclohexane liquid Extraction of Beta Carotene Beta carotene UV -

Tin-Free Alternatives to the Barton-Mccombie Deoxygenation of Alcohols to Alkanes Involving Reductive Electron Transfer
The French connecTion CHIMIA 2016, 70, No. 1/2 67 doi:10.2533/chimia.2016.67 Chimia 70 (2016) 67–76 © Swiss Chemical Society Tin-free Alternatives to the Barton- McCombie Deoxygenation of Alcohols to Alkanes Involving Reductive Electron Transfer Ludwig Chenneberg and Cyril Ollivier* Abstract: Echoing the recent celebration of the fortieth anniversary of the Barton-McCombie reaction, this review aims to explore another facet of radical processes for deoxygenation of alcohols by considering SET (single electron transfer) reduction of carboxylic ester, thiocarbonate and thiocarbamate derivatives. Various protocols have been developed relying on the use of organic and organometallic SET reagents, electrochemi- cal conditions, photoinduced electron transfer processes and visible-light photoredox catalysis. Applications to the synthesis of molecules of interest provide a glimpse into the scope of these different approaches. Keywords: Alcohols · Deoxygenation · Electron transfer · Radicals · Reduction 1. Introduction phorus derivatives and less toxic metals.[1] secondary and tertiary alcohols.[7] In 2015, Interesting perspectives have opened up the radical community celebrated the 40th Radical chemistry has witnessed an ex- with the use of organoboranes[4] and com- anniversary of the discovery of the Barton- plosive growth over the last three decades. pounds responsible for electron-transfer McCombie deoxygenation reaction, unan- Owing to their mildness and high com- reactions have been increasingly seen as imously considered as the most common -

Ep 3048090 A1
(19) TZZ¥ZZZ_T (11) EP 3 048 090 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 27.07.2016 Bulletin 2016/30 C07C 29/141 (2006.01) C07C 29/151 (2006.01) C07C 29/153 (2006.01) (21) Application number: 14845165.1 (86) International application number: (22) Date of filing: 17.09.2014 PCT/KR2014/008666 (87) International publication number: WO 2015/041471 (26.03.2015 Gazette 2015/12) (84) Designated Contracting States: •KIM,Jin Soo AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Daejeon 305-738 (KR) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • CHOE, Yong Jin PL PT RO RS SE SI SK SM TR Daejeon 305-738 (KR) Designated Extension States: • CHOI, Sun Hyuk BA ME Daejeon 305-738 (KR) (30) Priority: 17.09.2013 KR 20130111558 (74) Representative: Goddar, Heinz J. Boehmert & Boehmert (71) Applicant: LG Chem, Ltd. Anwaltspartnerschaft mbB Seoul 150-721 (KR) Patentanwälte Rechtsanwälte Pettenkoferstrasse 20-22 (72) Inventors: 80336 München (DE) • NAM, Hyun Daejeon 305-738 (KR) (54) METHOD FOR PREPARING ALKANOL (57) Provided are a method of preparing an alkanol and a device for preparing the same. According to the method and device, economic feasibility and stability of a preparation process may be enhanced, and mass production of an alkanol may be performed. EP 3 048 090 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 048 090 A1 Description [Technical Field] 5 [0001] The present application relates to a method of preparing an alkanol and a device for preparing the same.