Stereospecific Polymerization of Methacrylates with Ethylmagnesium Alkoxides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Improved Procedure for the Reductive Acetylation of Acyclic Esters and a New Synthesis of Ethers
J. Org. Chem. 2000, 65, 191-198 191 Improved Procedure for the Reductive Acetylation of Acyclic Esters and a New Synthesis of Ethers David J. Kopecky and Scott D. Rychnovsky* Department of Chemistry, University of California, Irvine, California 92717-2025 Received September 14, 1999 An optimized protocol for the DIBALH reductive acetylation of acyclic esters and diesters is described. This reductive acetylation procedure allows a wide variety of esters to be converted into the corresponding R-acetoxy ethers in good to excellent yields. It was found that, under mild acidic conditions, many R-acetoxy ethers can be further reduced to the corresponding ethers. This net two-step ester deoxygenation is an attractive alternative to the classical Williamson synthesis for certain ethers. Introduction The reduction and in situ acetylation of esters was developed in our laboratory several years ago to provide access to unusual cyclic acetal structures.1 The general strategy involved trapping of the aluminum hemiacetal intermediate found in the reduction of an ester to an aldehyde by diisobutylaluminum hydride (DIBALH). Other groups had previously trapped the same type of intermediate with a trimethylsilyl group.2 After some exploration, we found that acetic anhydride, DMAP, and pyridine led to efficient trapping to give R-acetoxy ethers 1 Figure 1. Original DIBALH reductive acetylation conditions from the cyclic esters we had been studying. We were for lactones and some acyclic esters. surprised to find that the same conditions gave satisfac- R tory yields of the -acetoxy ethers from acyclic esters. a 12 h period, an R-acetoxy ether of the general structure The R-acetoxy ethers can be activated with a variety of 3 was isolated. -

Identification of Promising Alternative Mono-Alcohol Fuel Blend
energies Article Identification of Promising Alternative Mono-Alcohol Fuel Blend Components for Spark Ignition Engines Saeid Aghahossein Shirazi 1, Thomas D. Foust 1,2 and Kenneth F. Reardon 1,2,* 1 Department of Chemical and Biological Engineering, Colorado State University, Fort Collins, CO 80523, USA; [email protected] (S.A.S.); [email protected] (T.D.F.) 2 National Renewable Energy Laboratory, Golden, CO 80401, USA * Correspondence: [email protected] Received: 24 March 2020; Accepted: 12 April 2020; Published: 15 April 2020 Abstract: Alcohols are attractive fuel blendstocks for spark ignition engines due to their high octane values and potentially positive influence on performance and emission. Although methanol, ethanol, and butanol have been widely studied, other biomass-derived alcohols may have similar or better properties. However, it is not feasible to experimentally investigate the fuel potential of every molecule. The goals of this study were to develop a methodology for rapid screening of a fuel property database for mono-alcohols and to identify alcohols with the potential of blending to produce advantaged motor gasolines. A database was developed with 13 fuel properties of all saturated C1–C10 mono-alcohols. A decision framework was used to evaluate alcohols suitable for blending in gasoline for spark ignition engines in two scenarios: low-range (up to 15 vol%) blends and high-range (greater than 40 vol%) blends. The low-range blend cases resulted in the identification of 48 alcohols. In the case of high-range blending, only six alcohols were found to be suitable. This is the first study to systematically evaluate all C1–C10 saturated alcohols for blending with gasoline using relevant fuel properties. -

Exam 1 (February 23, 2004) ID# ______
Chemistry 211 Name ___________________________ Exam 1 (February 23, 2004) ID# ___________________________ 10 1. You desire to synthesize 3-ethyl-3-pentanol starting with an ester. (i) What would be the name of the ester, and what is the name for the Grignard reagent (e.g., methyl magnesium bromide)? (ii) For the carbons shown in the product, show plausible hydrocarbons that you could start with to produce the ester and the Grignard reagent (as in a retrosynthesis). 12 2. (i) Show the step-by-step process required to produce propyllithium, which requires a free radical reaction mechanism, . (ii) Show the complete reaction mechanism for reaction between propyllithium and the correct ketone to produce 3-propyl-3-pentanol. (iii) Propose a possible reaction mechanism by which dipropyl cuprate (Cu+ with two propyl groups attached) could react with ethyl bromide to produce a new hydrocarbon. (This is a thinking exercise! So, think! () 8 3. As mentioned in the text, diethyl ether, pentane, and 1-butanol have similar molar masses, but different physical properties. Boiling points are 35oC, 36oC, and 117oC, respectively. Their respective solubilities in water are 7.5g/100mL, insoluble, and 9g/100mL. (i) Draw structures for each of these compounds. (ii) Justify the observed boiling points and their solubilities. 16 4. Draw structures of the following compounds 2,3-heptanediol isopropyllithium benzylmagnesium bromide benzoic acid benzaldehylde dimethyl sulfide t-butyl methanoate dibutyl ketone 12 5. Alcohols can be oxidized to produce other compounds, and can be produced by reduction. For the reactions shown below, show the structure for the expected product (if reaction does not occur, state: No Reaction) when treated with the indicated oxidizing or reducing agents. -

Catalog Feb 2007 Final.Pmd
Table of Contents How To Use This Catalog ............................................................................................... 2 Service Information .................................................................................................... 3-4 Placing Orders - Shipping........................................................................................................3 Miscellaneous Information......................................................................................................4 Product Information................................................................................................. 5-17 Definitions .............................................................................................................. 5 Detergent Analysis ............................................................................................. 6-7 Lipid-like Detergents.......................................................................................... 8-9 FOS-CHOLINE® Detergents .............................................................................................8 FOS-MEA® ...........................................................................................................................9 Miscellaneous ....................................................................................................................9 Nonionic Detergents ......................................................................................10-14 Sugar-based Detergents ....................................................................................... -

Catalytic Effect of Ultrananocrystalline Fe3o4 on Algal Bio-Crude Production Via HTL Process
Electronic Supplementary Material (ESI) for Nanoscale. This journal is © The Royal Society of Chemistry 2015 Catalytic Effect of Ultrananocrystalline Fe3O4 on Algal Bio-Crude Production via HTL Process Arnulfo Rojas-Péreza, Daysi Diaz-Diestraa,b, Cecilia B. Frias-Floresa, Juan Beltran-Huaraca,b, K. C. Dasc, Brad R. Weinera,b, Gerardo Morella,b and Liz M. Díaz-Vázquez*a Table 1-S: Profile of volatile and semivolatile compounds of the bio-crudes obtained from raw Ulva fasciata in absence of UNCFO (only compounds with quality identification factor higher that 70 were considered through NIST library) Library/ID Area Benzenepropanoic acid 0.1005 1H-Pyrrole 0.0114 Anthracene 0.0409 2,3,4-Trimethylpyrrole 0.0231 2,5-di-tert-Butyl-1,4-benzoquinone 0.0565 1H-Pyrrole 0.0493 Methylhydroquinon 0.1455 Pyridine 0.084 Acetophenone 0.0671 2-Pyridinamine, 4,6-dimethyl- 0.0094 Acetamide 0.08 2-Acetonylcyclopentanone 0.0097 1H-Indole, 2,6-dimethyl- 0.2334 Phenol 0.0503 1,2-Benzenediol 0.1657 Benzoic acid 0.09 Butylated Hydroxytoluene 0.2888 Benzenamine 0.0154 2-Isopropyl-10-methylphenanthrene 0.1123 2-Cyclopenten-1-one 0.147 5-Chloro-3-phenyl-1H-indazole 0.0976 2,4-Heptadiene 0.1024 1-Isopropyl-1H-indole 0.1426 Nonanoic acid 0.0385 Anthracene 0.067 7-Methylindan-1-one 0.0168 2,5-Diphenyl-2,4-hexadiene 0.0685 3-Methylcatechol 0.1156 2,3,7-Trimethylindole 0.2276 Acetophenone 0.1829 2-Methyl-5-(hexyn-1-yl)pyridine 0.0527 1H-Indole 0.1785 1-Nonadecene 0.2075 Acetamide, 0.1709 Heptadecanoic acid 0.2635 2,4-Diamino-N,N,5-trimethyl-6- 0.2219 9-Octadecenoic acid -

Grignard Reaction: Synthesis of Triphenylmethanol
*NOTE: Grignard reactions are very moisture sensitive, so all the glassware in the reaction (excluding the work-up) should be dried in an oven with a temperature of > 100oC overnight. The following items require oven drying. They should be placed in a 150mL beaker, all labeled with a permanent marker. 1. 5mL conical vial (AKA: Distillation receiver). 2. Magnetic spin vane. 3. Claisen head. 4. Three Pasteur pipettes. 5. Two 1-dram vials (Caps EXCLUDED). 6. One 2-dram vial (Caps EXCLUDED). 7. Glass stirring rod 8. Adaptor (19/22.14/20) Grignard Reaction: Synthesis of Triphenylmethanol Pre-Lab: In the “equations” section, besides the main equations, also: 1) draw the equation for the production of the byproduct, Biphenyl. 2) what other byproduct might occur in the reaction? Why? In the “observation” section, draw data tables in the corresponding places, each with 2 columns -- one for “prediction” (by answering the following questions) and one for actual drops or observation. 1) How many drops of bromobenzene should you add? 2) How many drops of ether will you add to flask 2? 3) 100 µL is approximately how many drops? 4) What are the four signs of a chemical reaction? (Think back to Chem. 110) 5) How do the signs of a chemical reaction apply to this lab? The Grignard reaction is a useful synthetic procedure for forming new carbon- carbon bonds. This organometallic chemical reaction involves alkyl- or aryl-magnesium halides, known as Grignard 1 reagents. Grignard reagents are formed via the action of an alkyl or aryl halide on magnesium metal. -

Organic Chemistry Laboratory II Preparation of Triphenylmethanol (Grignard Reaction) Experiment Procedure (Printable Pdf Format)
grigdes http://www.organicchem.org/oc2web/lab/exp/grig/grigdesbenzophenone... Organic Chemistry II | Lecture | Laboratory Organic Chemistry Laboratory II Preparation of Triphenylmethanol (Grignard Reaction) Experiment Procedure (Printable pdf format) Introduction In this two-week experiment, triphenylmethanol will be synthesized through a Grignard reaction. Students will work individually to prepare the Grignard reagent by reacting bromobenzene with solid magnesium in diethylether. The prepared Grignard reagent, phenylmagnesium bromide, will then be combined with bezophenone to form the desired triphenylmethanol product. Procedure NOTE: All glassware must be extremely dry! Place glassware in the drying oven for at least 10 minutes before beginning the reaction. Preparation of the Grignard Reagent Add ~0.5g of magnesium turnings and a magnetic stir bar to a dry 100 ml round-bottomed flask, and place the flask in the drying oven for 30 minutes. Remove the flask from the oven, clamp it to a ring stand, insert a reflux condenser, fit the flask into a heating mantle and immediately attach a drying tube to the top of the reflux condenser. Position the flask in the heating mantle on a stirrer/hotplate. Set the reaction up on the side of the hood near to the faucet labeled for distillation. Allow the reaction flask to cool to room temperature completely before proceeding. While the flask is cooling, place a 150ml beaker into the drying oven. While the flask is cooling, add 3.5 g of dry bromobenzene to a dry 50 mL Erlenmeyer flask and dissolve it in 5.0 mL of anhydrous diethyl ether. Use a glass powder funnel to dispense the bromobenze and ether to the Erlenmyer flask. -
![United States Patent [191 [11] Patent Number: 5,070,175 Tsumura Et Al](https://docslib.b-cdn.net/cover/3510/united-states-patent-191-11-patent-number-5-070-175-tsumura-et-al-583510.webp)
United States Patent [191 [11] Patent Number: 5,070,175 Tsumura Et Al
United States Patent [191 [11] Patent Number: 5,070,175 Tsumura et al. [45] Date of Patent: Dec. 3, 1991 [54] METHOD FOR THE PREPARATION OF AN Primary Examiner-Morton Foelak ORGANOPOLYSILOXANE CONTAINING Attorney, Agent, or Firm-Millen, White & Zelano TETRAFUNCI'IONAL SILOXANE UNITS [57] ABSTRACT [75] Inventors: Hiroshi Tsumura; Kiyoyuki Mutoh, An ef?cient and economically advantageous method is both of Gunma; Kazushi Satoh, proposed for the preparation of an organopolysiloxane Tokyo; Ken-ichi Isobe, Gunma, all of comprising tetrafunctional siloxane units, i.e. Q units, Japan and, typically, monofunctional siloxy units, i.e. M units, [73] Assignee: Shin-Etsu Chemical Co., Ltd., Tokyo, and useful as a reinforcing agent in silicone rubbers. The Japan method comprises the steps of: mixing the reactants for providing the Q and M units, such as ethyl orthosilicate [21] Appl. No.;. 706,148 and trimethyl methoxy silane, in a desired molar ratio; [22] Filed: May 28, 1991 and heating the mixture at a temperature higher by at least 10° C. than the boiling point of the mixture under [30] Foreign Application Priority Data normal pressure in a closed vessel in the presence of May 29, 1990 [JP] Japan ............ .Q .................. .. 2-l39ll9 water and a catalyst such as a sulfonic acid group-con taining compound. In addition to the greatly shortened [51] Int. 01.5 ............................................ .. C08G 77/06 reaction time and remarkably decreased contents of [52] U.S. c1. ...................................... .. 528/12; 528/10; residual alkoxy groups and gelled matter in the product, 528/21; 528/23; 528/34; 528/36 the method is advantageous also in respect of the ab [58] Field of Search .................... -

Chem 353 Derivative Tables
ALCOHOLS Derivatives Mp/ oC Compound Bp /oC 3,5-Dinitro- Phenylurethane Naphthyl- (Mp / oC) benzoate urethane 4-Methylbenzyl alcohol --- (60) 118 79 --- Diphenylmethanol --- (69) 141 136 --- Ethyl p-hydroxybenzoate --- (116) * * * Methyl p-hydroxybenzoate --- (130) * * * Ethanol 78 93 52 79 2-Propanol 83 122 88 106 1-Propanol 97 74 51 80 2-Butanol 99 75 65 97 2-Methyl-1-propanol 108 86 86 104 3-Methyl-2-butanol 113 76 68 110 1-Butanol 116 64 63 71 3-Pentanol 116 101 48 95 2-Pentanol 119 61 --- (oil) 76 2-Chloroethanol 129 92 51 101 2-Methyl-1-butanol 129 70 31 82 3-Methyl-1-butanol 132 61 55 68 4-Methyl-2-pentanol 132 65 143 88 3-Methyl-2-pentanol 134 43 --- 72 1-Pentanol 138 46 46 68 2-Methyl-1-pentanol 148 51 --- 75 2-Ethyl-1-butanol 149 52 --- --- 1-Hexanol 156 58 42 59 Cyclohexanol 161 112 82 129 2-Octanol 179 32 114 63 1-Phenylethanol 203 95 94 106 Benzyl alcohol 205 112 78 134 2-Phenylethanol 219 108 79 119 Ethyl o-hydroxybenzoate 234 * * * Cinnamyl alcohol 257 (33) 121 90 114 4-Methoxybenzyl alcohol 260 (25) --- 92 --- * These compounds are also esters, see that table for derivatives. --- No data available. ESTERS Derivative Mp/ oC Compound Bp /oC Carboxylic (Mp / oC) Acid Methyl p-methylbenzoate 223 (30) 177 Methyl cinnamate 261 (35) 133 Methyl 4-methoxybenzoate 245 (49) 184 Ethyl p-nitrobenzoate --- (57) 241 Methyl p-nitrobenzoate --- (95) 241 Ethyl p-hydroxybenzoate --- (116) 213 Methyl p-hydroxybenzoate --- (130) 213 Methyl benzoate 198 121 Methyl o-toluate 213 102 Ethyl benzoate 213 121 Diethyl succinate 216 190 Methyl phenylacetate 218 76 Methyl salicylate 224 157 Methyl o-chlorobenzoate 230 140 Ethyl salicylate 234 157 Ethyl p-toluate 241 177 Isopropyl salicylate 255 157 Ethyl o-chlorobenzoate 255 140 Dimethyl suberate 268 141 Ethyl 4-methoxybenzoate 270 184 Ethyl cinnamate 271 133 Diethyl phthalate 296 230 --- No data available. -

Ch3ch2cch(Ch3)2 O Ch2ch2cho Ch3cch2ch2ch2cch2ch3
Chem 226 — Problem Set #9 — “Fundamentals of Organic Chemistry,” 4th edition, John McMurry. Chapter 9 2. Name the following aldehydes and ketones. O (a) (b) CH2CH2CHO CH3CH2CCH(CH3)2 H CH3 (c) O O (d) H C O CH3CCH2CH2CH2CCH2CH3 H (a) 2-methyl-3-pentanone, (b) 3-phenylpropanal, (c) 2,6-octanedione, (d) (1R,2R)-2-methylcyclohexanecarbaldehyde 4. How could you prepare pentanal from the following starting materials? (a) 1-pentanol, (b) CH3CH2CH2CH2COOH, (c) 5-decene PCC (a) CH3CH2CH2CH2CH2OH CH3CH2CH2CH2CHO CH2Cl2 O 1. LiAlH4 CH3CH2CH2CH2C OH CH3CH2CH2CH2CH2OH H O+ (b) 2. 3 PCC CH3CH2CH2CH2CH2OH CH3CH2CH2CH2CHO CH2Cl2 KMnO4 CH3CH2CH2CH2CH CHCH2CH2CH2CH3 + H3O O LiAlH (c) 1. 4 CH3CH2CH2CH2C OH + CH3CH2CH2CH2CH2OH 2. H3O PCC CH3CH2CH2CH2CH2OH CH3CH2CH2CH2CHO CH2Cl2 5. How could you prepare 2-hexanone from the following starting materials? (a) 2-hexanol, (b) 1-hexyne, (c) 2-methyl-1-hexene OH O K2CrO7 (a) CH3CH2CH2CH2CHCH3 CH3CH2CH2CH2CCH3 O H2SO4 (b) CH3CH2CH2CH2C CH CH3CH2CH2CH2CCH3 HgSO4 H2O CH3 O KMnO4 (c) CH3CH2CH2CH2C CH2 CH3CH2CH2CH2CCH3 + H3O (b) The enol that initially forms here undergoes keto-enol tautomerism. Terminal alkynes give methyl ketones; they follow Markovnikov’s rule. (c) Potassium permanganate under basic conditions would hydroxylate the alkene to form a vicinal diol, but under acidic conditions it completely cleaves the double bond. The =CH2 group would become carbon dioxide. 6. How would you carry out the following transformations? More than one step may be required. (a) 3-hexene 3-hexanone (b) benzene 1-phenylethanol H OH H2O (a) CH3CH2CH CHCH2CH3 CH3CH2CH CHCH2CH3 H SO 2 4 H O K2Cr2O7 CH3CH2CH CCH2CH3 O CH3CCl (b) C CH3 AlCl3 O H NaBH4 C CH3 OH 9. -

USP Excipient Reference Standards Catalog
Last Updated On: March 15, 2021 USP Excipient Reference Standards Catalog Catalog # Description Current Lot Previous CAS # NDC # Unit Price Special Restriction Lot(Valid Use Date) 1002505 Acesulfame G0H082 F0C136 (30-JUN- 55589-62-3 N/A $410.00 Potassium (200 mg) 2009) 1005706 Glacial Acetic Acid R038A0 I0M342 (31-JUL- 64-19-7 N/A $280.00 (1.5 mL/ampule; 3 2017) ampules) 1006801 Acetone (1.5 R095B0 I0M548 (28-FEB- 67-64-1 N/A $265.00 mL/ampule; 3 2022) ampules) 1009005 Acetylcysteine (200 R10200 K0K294 (30- 616-91-1 N/A $245.00 mg) NOV-2019) 1009901 Acetyltributyl Citrate R053A0 H0I337 (31-AUG- 77-90-7 N/A $275.00 (3 x 200 mg) 2017) 1009923 Acetyltriethyl Citrate R041M0 H0I339 (31-JAN- 77-89-4 N/A $275.00 (500 mg) 2017) 1012190 Adipic Acid (100 mg) F1D318 F0D318 (31- 124-04-9 N/A $265.00 MAR-2018) 1012214 Agar (500 mg) F0K137 N/A N/A $253.00 1012595 rAlbumin Human (6 R04600 G0M268 (31- N/A N/A $314.00 Cold Shipment mg) (Recombinant DEC-2016) Required Human Albumin) (COLD SHIPMENT REQUIRED) 1012688 Alcohol R05900 G0M024 (31- 64-17-5 N/A $265.00 Determination-- OCT-2018) Alcohol (5 Page 1 Last Updated On: March 15, 2021 USP Excipient Reference Standards Catalog Catalog # Description Current Lot Previous CAS # NDC # Unit Price Special Restriction Lot(Valid Use Date) mL/ampule; 5 ampules) 1012768 Alcohol (1.2 R127S0 R087W0 (31- 64-17-5 N/A $245.00 mL/ampule; 5 MAY-2020) ampules) 1012772 Dehydrated Alcohol R119J0 G0M292 (31- 64-17-5 N/A $245.00 (1.2 mL/ampule; 5 MAY-2020) ampules) 1012799 Aleuritic Acid (50 F02300 533-87-9 N/A $278.00 mg) -
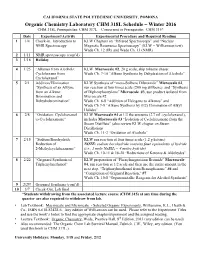
318 Lab Schedule
CALIFORNIA STATE POLYTECHNIC UNIVERSITY, POMONA Organic Chemistry Laboratory CHM 318L Schedule – Winter 2016 CHM 318L Prerequisites: CHM 317L Concurrent or Prerequisite: CHM 315* Date Experiment/Activity Experimental Procedure and Required Reading 1 1/4 Check-in. Introduction to KLW Chapters on “Infrared Spectroscopy” and “Nuclear NMR Spectroscopy Magnetic Resonance Spectroscopy” (KLW = Williamson text) Wade Ch. 12 (IR) and Wade Ch. 13 (NMR) 2 1/11 NMR spectroscopy (cont’d) 3 1/18 Holiday 4 1/25 “Alkenes from Alcohols: KLW Macroscale #2, 20 g scale, skip toluene chaser Cyclohexene from Wade Ch. 7-10 “Alkene Synthesis by Dehydration of Alcohols” Cyclohexanol” 5 2/1 Addition/Elimination KLW Synthesis of “meso-Stilbene Dibromide” Microscale #2, “Synthesis of an Alkyne run reaction at four times scale (200 mg stillbene); and “Synthesis from an Alkene: of Diphenylacetylene” Microscale #3, use product isolated from Bromination and Microscale #2 Dehydrobromination” Wade Ch. 8-8 “Addition of Halogens to Alkenes” and Wade Ch 7-9 “Alkene Synthesis by (E2) Elimination of Alkyl Halides” 6 2/8 “Oxidation: Cyclohexanol KLW Macroscale #4 at 1/3 the amounts (2.7 mL cyclohexanol), to Cyclohexanone” includes Macroscale #3 “Isolation of Cyclohexanone from the Steam Distillate” (also review KLW chapter on Steam Distillation) Wade Ch. 11-2 “Oxidation of Alcohols” 7 2/15 “Sodium Borohydride KLW run reaction at four times scale (1.2 g ketone) Reduction of NOTE: sodium borohydride contains four equivalents of hydride 2-Methylcyclohexanone” (i.e., 1 mole NaBH4 = 4 moles hydride) Wade Ch. 10-11 & 18-20 “Reductions of Ketones & Aldehydes” 8 2/22 “Grignard Synthesis of KLW preparation of “Phenylmagnesium Bromide” Macroscale Triphenylmethanol” #4, run reaction at 1/2 scale and then use the entire amount in the next step, “Triphenylmethanol from Benzophenone” #6 and “Completion of Grignard Reaction,” #7 Wade Ch.