(Title of the Thesis)*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Disease List for Aquaculture Health Certificate
Quarantine Standard for Designated Species of Imported/Exported Aquatic Animals [Attached Table] 4. Listed Diseases & Quarantine Standard for Designated Species Listed disease designated species standard Common name Disease Pathogen 1. Epizootic haematopoietic Epizootic Perca fluviatilis Redfin perch necrosis(EHN) haematopoietic Oncorhynchus mykiss Rainbow trout necrosis virus(EHNV) Macquaria australasica Macquarie perch Bidyanus bidyanus Silver perch Gambusia affinis Mosquito fish Galaxias olidus Mountain galaxias Negative Maccullochella peelii Murray cod Salmo salar Atlantic salmon Ameirus melas Black bullhead Esox lucius Pike 2. Spring viraemia of Spring viraemia of Cyprinus carpio Common carp carp, (SVC) carp virus(SVCV) Grass carp, Ctenopharyngodon idella white amur Hypophthalmichthys molitrix Silver carp Hypophthalmichthys nobilis Bighead carp Carassius carassius Crucian carp Carassius auratus Goldfish Tinca tinca Tench Sheatfish, Silurus glanis European catfish, wels Negative Leuciscus idus Orfe Rutilus rutilus Roach Danio rerio Zebrafish Esox lucius Northern pike Poecilia reticulata Guppy Lepomis gibbosus Pumpkinseed Oncorhynchus mykiss Rainbow trout Abramis brama Freshwater bream Notemigonus cysoleucas Golden shiner 3.Viral haemorrhagic Viral haemorrhagic Oncorhynchus spp. Pacific salmon septicaemia(VHS) septicaemia Oncorhynchus mykiss Rainbow trout virus(VHSV) Gadus macrocephalus Pacific cod Aulorhynchus flavidus Tubesnout Cymatogaster aggregata Shiner perch Ammodytes hexapterus Pacific sandlance Merluccius productus Pacific -

(Sea of Okhotsk, Sakhalin Island): 2. Cyclopteridae−Molidae Families
ISSN 0032-9452, Journal of Ichthyology, 2018, Vol. 58, No. 5, pp. 633–661. © Pleiades Publishing, Ltd., 2018. An Annotated List of the Marine and Brackish-Water Ichthyofauna of Aniva Bay (Sea of Okhotsk, Sakhalin Island): 2. Cyclopteridae−Molidae Families Yu. V. Dyldina, *, A. M. Orlova, b, c, d, A. Ya. Velikanove, S. S. Makeevf, V. I. Romanova, and L. Hanel’g aTomsk State University (TSU), Tomsk, Russia bRussian Federal Research Institute of Fishery and Oceanography (VNIRO), Moscow, Russia cInstitute of Ecology and Evolution, Russian Academy of Sciences (IPEE), Moscow, Russia d Dagestan State University (DSU), Makhachkala, Russia eSakhalin Research Institute of Fisheries and Oceanography (SakhNIRO), Yuzhno-Sakhalinsk, Russia fSakhalin Basin Administration for Fisheries and Conservation of Aquatic Biological Resources—Sakhalinrybvod, Aniva, Yuzhno-Sakhalinsk, Russia gCharles University in Prague, Prague, Czech Republic *e-mail: [email protected] Received March 1, 2018 Abstract—The second, final part of the work contains a continuation of the annotated list of fish species found in the marine and brackish waters of Aniva Bay (southern part of the Sea of Okhotsk, southern part of Sakhalin Island): 137 species belonging to three orders (Perciformes, Pleuronectiformes, Tetraodon- tiformes), 31 family, and 124 genera. The general characteristics of ichthyofauna and a review of the commer- cial fishery of the bay fish, as well as the final systematic essay, are presented. Keywords: ichthyofauna, annotated list, conservation status, commercial importance, marine and brackish waters, Aniva Bay, southern part of the Sea of Okhotsk, Sakhalin Island DOI: 10.1134/S0032945218050053 INTRODUCTION ANNOTATED LIST OF FISHES OF ANIVA BAY The second part concludes the publication on the 19. -

Identification of Larvae of Three Arctic Species of Limanda (Family Pleuronectidae)
Identification of larvae of three arctic species of Limanda (Family Pleuronectidae) Morgan S. Busby, Deborah M. Blood & Ann C. Matarese Polar Biology ISSN 0722-4060 Polar Biol DOI 10.1007/s00300-017-2153-9 1 23 Your article is protected by copyright and all rights are held exclusively by 2017. This e- offprint is for personal use only and shall not be self-archived in electronic repositories. If you wish to self-archive your article, please use the accepted manuscript version for posting on your own website. You may further deposit the accepted manuscript version in any repository, provided it is only made publicly available 12 months after official publication or later and provided acknowledgement is given to the original source of publication and a link is inserted to the published article on Springer's website. The link must be accompanied by the following text: "The final publication is available at link.springer.com”. 1 23 Author's personal copy Polar Biol DOI 10.1007/s00300-017-2153-9 ORIGINAL PAPER Identification of larvae of three arctic species of Limanda (Family Pleuronectidae) 1 1 1 Morgan S. Busby • Deborah M. Blood • Ann C. Matarese Received: 28 September 2016 / Revised: 26 June 2017 / Accepted: 27 June 2017 Ó Springer-Verlag GmbH Germany 2017 Abstract Identification of fish larvae in Arctic marine for L. proboscidea in comparison to the other two species waters is problematic as descriptions of early-life-history provide additional evidence suggesting the genus Limanda stages exist for few species. Our goal in this study is to may be paraphyletic, as has been proposed in other studies. -

Pleuronectidae
FAMILY Pleuronectidae Rafinesque, 1815 - righteye flounders [=Heterosomes, Pleronetti, Pleuronectia, Diplochiria, Poissons plats, Leptosomata, Diprosopa, Asymmetrici, Platessoideae, Hippoglossoidinae, Psettichthyini, Isopsettini] Notes: Hétérosomes Duméril, 1805:132 [ref. 1151] (family) ? Pleuronectes [latinized to Heterosomi by Jarocki 1822:133, 284 [ref. 4984]; no stem of the type genus, not available, Article 11.7.1.1] Pleronetti Rafinesque, 1810b:14 [ref. 3595] (ordine) ? Pleuronectes [published not in latinized form before 1900; not available, Article 11.7.2] Pleuronectia Rafinesque, 1815:83 [ref. 3584] (family) Pleuronectes [senior objective synonym of Platessoideae Richardson, 1836; family name sometimes seen as Pleuronectiidae] Diplochiria Rafinesque, 1815:83 [ref. 3584] (subfamily) ? Pleuronectes [no stem of the type genus, not available, Article 11.7.1.1] Poissons plats Cuvier, 1816:218 [ref. 993] (family) Pleuronectes [no stem of the type genus, not available, Article 11.7.1.1] Leptosomata Goldfuss, 1820:VIII, 72 [ref. 1829] (family) ? Pleuronectes [no stem of the type genus, not available, Article 11.7.1.1] Diprosopa Latreille, 1825:126 [ref. 31889] (family) Platessa [no stem of the type genus, not available, Article 11.7.1.1] Asymmetrici Minding, 1832:VI, 89 [ref. 3022] (family) ? Pleuronectes [no stem of the type genus, not available, Article 11.7.1.1] Platessoideae Richardson, 1836:255 [ref. 3731] (family) Platessa [junior objective synonym of Pleuronectia Rafinesque, 1815, invalid, Article 61.3.2 Hippoglossoidinae Cooper & Chapleau, 1998:696, 706 [ref. 26711] (subfamily) Hippoglossoides Psettichthyini Cooper & Chapleau, 1998:708 [ref. 26711] (tribe) Psettichthys Isopsettini Cooper & Chapleau, 1998:709 [ref. 26711] (tribe) Isopsetta SUBFAMILY Atheresthinae Vinnikov et al., 2018 - righteye flounders GENUS Atheresthes Jordan & Gilbert, 1880 - righteye flounders [=Atheresthes Jordan [D. -

Ÿþø R J a N H a G E N P H D T H E S
MUSCLE GROWTH AND FLESH QUALITY OF FARMED ATLANTIC HALIBUT (HIPPOGLOSSUS HIPPOGLOSSUS) IN RELATION TO SEASON OF HARVEST Ørjan Hagen A Thesis Submitted for the Degree of PhD at the University of St. Andrews 2008 Full metadata for this item is available in the St Andrews Digital Research Repository at: https://research-repository.st-andrews.ac.uk/ Please use this identifier to cite or link to this item: http://hdl.handle.net/10023/642 This item is protected by original copyright Muscle growth and flesh quality of farmed Atlantic halibut (Hippoglossus hippoglossus) in relation to season of harvest. Ørjan Hagen A thesis submitted for the degree of Doctor of Philosophy University of St Andrews St Andrews, July 2008 ii This thesis is dedicated to my family. I could not have managed without their loving support. Thank you so much! iii Acknowledgements Taking a PhD abroad at a highly respected University across the world has been a milestone of my life. I am very proud to have been a PhD student at the University of St Andrews and very grateful to the people helping me reaching my goals. Looking back at the beginning (October 2003) these last few years have been an educational expedition, full of challenges and memories. I have met people from Australia and Taiwan in the east to Canada in the west, wonderful people that I hope to have collaboration with in the future. There are so many to thank, and I would like to start with my supervisor and the head of the “Fish Muscle Research Group” (FMRG), Prof. -

Humboldt Bay Fishes
Humboldt Bay Fishes ><((((º>`·._ .·´¯`·. _ .·´¯`·. ><((((º> ·´¯`·._.·´¯`·.. ><((((º>`·._ .·´¯`·. _ .·´¯`·. ><((((º> Acknowledgements The Humboldt Bay Harbor District would like to offer our sincere thanks and appreciation to the authors and photographers who have allowed us to use their work in this report. Photography and Illustrations We would like to thank the photographers and illustrators who have so graciously donated the use of their images for this publication. Andrey Dolgor Dan Gotshall Polar Research Institute of Marine Sea Challengers, Inc. Fisheries And Oceanography [email protected] [email protected] Michael Lanboeuf Milton Love [email protected] Marine Science Institute [email protected] Stephen Metherell Jacques Moreau [email protected] [email protected] Bernd Ueberschaer Clinton Bauder [email protected] [email protected] Fish descriptions contained in this report are from: Froese, R. and Pauly, D. Editors. 2003 FishBase. Worldwide Web electronic publication. http://www.fishbase.org/ 13 August 2003 Photographer Fish Photographer Bauder, Clinton wolf-eel Gotshall, Daniel W scalyhead sculpin Bauder, Clinton blackeye goby Gotshall, Daniel W speckled sanddab Bauder, Clinton spotted cusk-eel Gotshall, Daniel W. bocaccio Bauder, Clinton tube-snout Gotshall, Daniel W. brown rockfish Gotshall, Daniel W. yellowtail rockfish Flescher, Don american shad Gotshall, Daniel W. dover sole Flescher, Don stripped bass Gotshall, Daniel W. pacific sanddab Gotshall, Daniel W. kelp greenling Garcia-Franco, Mauricio louvar -

Maren Mommens Phd Thesis
MATERNAL EFFECTS ON OOCYTE QUALITY IN FARMED ATLANTIC HALIBUT (HIPPOGLOSSUS HIPPOGLOSSUS L.) Maren Mommens A Thesis Submitted for the Degree of PhD at the University of St Andrews 2012 Full metadata for this item is available in Research@StAndrews:FullText at: http://research-repository.st-andrews.ac.uk/ Please use this identifier to cite or link to this item: http://hdl.handle.net/10023/3661 This item is protected by original copyright Maternal effects on oocyte quality in farmed Atlantic halibut (Hippoglossus hippoglossus L.) Maren Mommens This thesis is submitted in partial fulfilment for the degree of PhD at the University of St Andrews September 2011 This thesis is dedicated to my parents. 2 Acknowledgements First of all, I would like to express my gratitude to my supervisors Professor Ian A. Johnston, Professor Igor Babiak and Dr. Jorge M.O. Fernandes for their invaluable support, advice and constructive feedback. My thanks go to Risør Fisk AS and especially Kjell E. Naas and Yngve Attramadal for their cooperation and help during sampling. The microarray collaboration with Dr. Knut Erik Tollefsen from the Norwegian Institute for Water Research (NIVA) was much appreciated and I would like to thank You Song for his excellent training in microarray preparation. The members of the Fish Muscle Research Group at the University of St. Andrews introduced me to molecular biology and I would especially like to thank Dr. Hung-Tai Lee and Dr. Sitheswarab Nainamalai for help and support in the lab. Dr. Neil Bower arrived at the same time as me in St.Andrews and together with his family we shared our Scotland experience. -

Bilateral Asymmetry and Bilateral Variation in Fishes *
BILATERAL ASYMMETRY AND BILATERAL VARIATION IN FISHES * bARL L. HUBBS AND LAURA C. HUBBS CONTENTS PAGE Introduction ................................................................................................................... 230 Statistical methods ....................................................................................................... 231 Dextrality and sinistrality in flatfishes .................................................................. 234 Reversal of sides in flounders .............................................................................. 236 Decreased viability of reversed flounders ......................................................... 240 Incomplete mirror imaging in reversed flounders .......................................... 243 A completely reversed flatfish .............................................................................. 245 Interpretation of reversal in flatfishes ............................................................... 246 Teratological return toward symmetry ............................................................. 249 Secondary asymmetries in flatfishes .................................................................... 250 Bilateral variation in number of rays in paired fins on the two sides of flatfishes ................................................................................................................. 254 Asymmetries and bilateral variations in essentially symmetrical fishes ....... 263 Bilateral variation in number of rays in the left -

AUSTRALIAN NATIONAL SPEARFISHING RECORDS JULY 2018 COMPILED by AUF RECORDS OFFICER VIN RUSHWORTH Common Names
AUSTRALIAN NATIONAL SPEARFISHING RECORDS JULY 2018 COMPILED BY AUF RECORDS OFFICER VIN RUSHWORTH Common Names Common Name Pg. Common Name Pg. Albacore 37 Cod, Barramundi 39 Amberjack 8 Cod, Bearded 27 Amberjack, High-fin 9 Cod, Black 40 Angelfish Yellow-Mask 32 Cod, Blacksaddle Rock 40 Angelfish, Blue 32 Cod, Blackspotted 40 Angelfish, Imperial 32 Cod, Black-Tipped Rock 40 Angelfish, Six Banded 32 Cod, Break-Sea 39 Anglerfish, Spinycoat 3 Cod, Camouflage 40 Barracouta 13 Cod, Chinaman 41 Barracuda, Blackfin 44 Cod, Coral 39 Barracuda, Blue and Gold 44 Cod, Coral Rock 39 Barracuda, Chevron 44 Cod, Dusky 42 Barracuda, Great 44 Cod, Flowery 40 Barracuda, Pickhandle 44 Cod, Freckled Coral 39 Barramundi 20 Cod, Gold Spotted 39 Bass, Red 23 Cod, Highfin 40 Batfish, Black-tip 13 Cod, Long-finned Rock 41 Batfish, Hump-headed 13 Cod, Long-headed 41 Batfish, Long-finned 13 Cod, Maori 41 Batfish, Long-snout 13 Cod, Masked 41 Batfish, Short-finned 13 Cod, Ocellated 39 Bigeye, Lunar-tailed 33 Cod, Peacock Coral 39 Blackfish, Banded Rock 14 Cod, Potato 41 Blackfish, Rock 14 Cod, Pug-Nosed Wire-netting 40 Blackfish, Western Rock 14 Cod, Purple 40 Blanquillo, Blue 25 Cod, Rankin 40 Blue Devil, Eastern 31 Cod, Red 27 Blue Devil, Southern 31 Cod, Red Rock 37 Bluefish 13 Cod, Red Rock Western 38 Blue-lined Seabream 24 Cod, Red-flushed Rock 38 Boarfish, Giant 30 Cod, Speckledfin 40 Boarfish, Longsnout 30 Cod, Tomato Coral 39 Boarfish, Short 30 Cod, Twin-Spot 39 Boarfish, Yellow-spotted 30 Cod, White-lined Rock 38 Bonefish, Eastern 3 Coral Fish, New-moon -

Japan Update to 05.04.2021 Approval No Name Address Products Number FROZEN CHUM SALMON DRESSED (Oncorhynchus Keta)
Japan Update to 05.04.2021 Approval No Name Address Products Number FROZEN CHUM SALMON DRESSED (Oncorhynchus keta). FROZEN DOLPHINFISH DRESSED (Coryphaena hippurus). FROZEN JAPANESE SARDINE ROUND (Sardinops 81,Misaki-Cho,Rausu- Kaneshin Tsuyama melanostictus). FROZEN ALASKA POLLACK DRESSED (Theragra chalcogramma). 1 VN01870001 Cho, Menashi- Co.,Ltd FROZEN ALASKA POLLACK ROUND (Theragra chalcogramma). FROZEN PACIFIC COD Gun,Hokkaido,Japan DRESSED. (Gadus macrocephalus). FROZEN PACIFIC COD ROUND. (Gadus macrocephalus) Maekawa Shouten Hokkaido Nemuro City Fresh Fish (Excluding Fish By-Product); Fresh Bivalve Mollusk.; Frozen Fish (Excluding 2 VN01860002 Co., Ltd Nishihamacho 10-177 Fish By-Product); Frozen Processed Bivalve Mollusk; Frozen Chum Salmon(Round,Dressed,Semi-Dressed,Fillet,Head,Bone,Skin); Frozen 1-35-1 Alaska Pollack(Round,Dressed,Semi-Dressed,Fillet); Frozen Pacific Taiyo Sangyo Co.,Ltd. 3 VN01840003 Showachuo,Kushiro- Cod(Round,Dressed,Semi-Dressed,Fillet); Frozen Pacific Saury(Round,Dressed,Semi- Kushiro Factory City,Hokkaido,Japan Dressed); Frozen Chub Mackerel(Round,Fillet); Frozen Blue Mackerel(Round,Fillet); Frozen Salted Pollack Roe 3-9 Komaba- Taiyo Sangyo Co.,Ltd. 4 VN01860004 Cho,Nemuro- Frozen Fish ; Frozen Processed Fish; (Excluding By-Product) Nemuro Factory City,Hokkaido,Japan 3-2-20 Kitahama- Marutoku Abe Suisan 5 VN01920005 Cho,Monbetu- Frozen Chum Salmon Dressed; Frozen Salmon Dressed Co.,Ltd City,Hokkaido,Japan Frozen Chum Salmon(Round,Semi-Dressed,Fillet); Frozen Salmon Milt; Frozen Pink Salmon(Round,Semi-Dressed,Dressed,Fillet); -
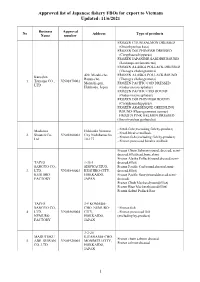
Approved List of Japanese Fishery Fbos for Export to Vietnam Updated: 11/6/2021
Approved list of Japanese fishery FBOs for export to Vietnam Updated: 11/6/2021 Business Approval No Address Type of products Name number FROZEN CHUM SALMON DRESSED (Oncorhynchus keta) FROZEN DOLPHINFISH DRESSED (Coryphaena hippurus) FROZEN JAPANESE SARDINE ROUND (Sardinops melanostictus) FROZEN ALASKA POLLACK DRESSED (Theragra chalcogramma) 420, Misaki-cho, FROZEN ALASKA POLLACK ROUND Kaneshin Rausu-cho, (Theragra chalcogramma) 1. Tsuyama CO., VN01870001 Menashi-gun, FROZEN PACIFIC COD DRESSED LTD Hokkaido, Japan (Gadus macrocephalus) FROZEN PACIFIC COD ROUND (Gadus macrocephalus) FROZEN DOLPHIN FISH ROUND (Coryphaena hippurus) FROZEN ARABESQUE GREENLING ROUND (Pleurogrammus azonus) FROZEN PINK SALMON DRESSED (Oncorhynchus gorbuscha) - Fresh fish (excluding fish by-product) Maekawa Hokkaido Nemuro - Fresh bivalve mollusk. 2. Shouten Co., VN01860002 City Nishihamacho - Frozen fish (excluding fish by-product) Ltd 10-177 - Frozen processed bivalve mollusk Frozen Chum Salmon (round, dressed, semi- dressed,fillet,head,bone,skin) Frozen Alaska Pollack(round,dressed,semi- TAIYO 1-35-1 dressed,fillet) SANGYO CO., SHOWACHUO, Frozen Pacific Cod(round,dressed,semi- 3. LTD. VN01840003 KUSHIRO-CITY, dressed,fillet) KUSHIRO HOKKAIDO, Frozen Pacific Saury(round,dressed,semi- FACTORY JAPAN dressed) Frozen Chub Mackerel(round,fillet) Frozen Blue Mackerel(round,fillet) Frozen Salted Pollack Roe TAIYO 3-9 KOMABA- SANGYO CO., CHO, NEMURO- - Frozen fish 4. LTD. VN01860004 CITY, - Frozen processed fish NEMURO HOKKAIDO, (excluding by-product) FACTORY JAPAN -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste