5Oe Non REPULPER -- FILTER
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Radiochemistry of Beryllium
National Academy of Sciences National Research Council I NUCLEAR SCIENCE SERIES The Radiochemistry ·of Beryllium COMMITTEE ON NUCLEAR SCIENCE L. F. CURTISS, Chairman ROBLEY D. EVANS, Vice Chairman National Bureau of Standards MassaChusetts Institute of Technol0gy J. A. DeJUREN, Secretary ./Westinghouse Electric Corporation H.J. CURTIS G. G. MANOV Brookhaven National' LaboratOry Tracerlab, Inc. SAMUEL EPSTEIN W. WAYNE MEINKE CalUornia Institute of Technology University of Michigan HERBERT GOLDSTEIN A.H. SNELL Nuclear Development Corporation of , oak Ridge National Laboratory America E. A. UEHLING H.J. GOMBERG University of Washington University of Michigan D. M. VAN PATTER E.D.KLEMA Bartol Research Foundation Northwestern University ROBERT L. PLATZMAN Argonne National Laboratory LIAISON MEMBERS PAUL C .. AEBERSOLD W.D.URRY Atomic Energy Commission U. S. Air Force J. HOW ARD McMILLEN WILLIAM E. WRIGHT National Science Foundation Office of Naval Research SUBCOMMITTEE ON RADIOCHEMISTRY W. WAYNE MEINKE, Chairman HAROLD KIRBY University of Michigan Mound Laboratory GREGORY R. CHOPPIN GEORGE LEDDICOTTE Florida State University. Oak Ridge National Laboratory GEORGE A. COW AN JULIAN NIELSEN Los Alamos Scientific Laboratory Hanford Laboratories ARTHUR W. FAIRHALL ELLIS P. STEINBERG University of Washington Argonne National Laboratory JEROME HUDIS PETER C. STEVENSON Brookhaven National Laboratory University of California (Livermore) EARL HYDE LEO YAFFE University of CalUornia (Berkeley) McGill University CONSULTANTS NATHAN BALLOU WILLIAM MARLOW Naval Radiological Defense Laboratory N atlonal Bureau of Standards JAMESDeVOE University of Michigan CHF.MISTRY-RADIATION AND RADK>CHEMIST The Radiochemistry of Beryllium By A. W. FAIRHALL. Department of Chemistry University of Washington Seattle, Washington May 1960 ' Subcommittee on Radiochemistry National Academy of Sciences - National Research Council Printed in USA. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/DK20 15/050343 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 11 November 2015 ( 11. 1 1.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: PA 2014 00655 11 November 2014 ( 11. 1 1.2014) DK (84) Designated States (unless otherwise indicated, for every 62/077,933 11 November 2014 ( 11. 11.2014) US kind of regional protection available): ARIPO (BW, GH, 62/202,3 18 7 August 2015 (07.08.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: LUNDORF PEDERSEN MATERIALS APS TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, [DK/DK]; Nordvej 16 B, Himmelev, DK-4000 Roskilde DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (DK). -

Beryllium and Beryllium Compounds
BERYLLIUM AND BERYLLIUM COMPOUNDS Beryllium and beryllium compounds were considered by previous IARC Working Groups in 1971, 1979, 1987, and 1993 (IARC, 1972, 1980, 1987, 1993). Since that time, new data have become available, these have been incorporated in the Monograph, and taken into consid- eration in the present evaluation. 1. Exposure Data a very high strength-to-weight ratio. Beryllium is lighter than aluminium but is greater than 40% 1.1 Identification of the agents more rigid than steel. It has excellent electrical and thermal conductivities. Its only markedly Synonyms and molecular formulae for beryl- adverse feature is relatively pronounced brittle- lium, beryllium–aluminium and beryllium– ness, which restricts the use of metallic beryl- copper alloys, and certain beryllium compounds lium to specialized applications (WHO, 1990). are presented in Table 1.1. The list is not exhaus- Because of its low atomic number, beryllium tive, nor does it comprise necessarily the most is very permeable to X-rays. Neutron emission commercially important beryllium-containing after bombardment with α or γ rays is the most substances; rather, it indicates the range of beryl- important of its nuclear physical properties, lium compounds available. and beryllium can be used as a neutron source. Moreover, its low neutron absorptiveness and high-scattering cross-section make it a suitable 1.2 Chemical and physical properties moderator and reflector in structural materials of the agents in nuclear facilities; where most other metals absorb neutrons emitted during the fission Beryllium (atomic number, 4; relative atomic of nuclear fuel, beryllium atoms only reduce mass, 9.01) is a metal, which belongs to Group the energy of such neutrons, and reflect them IIA of the Periodic Table. -

Attachment 3-1 Guidance for Developing Ecological Soil
Attachment 3-1 Guidance for Developing Ecological Soil Screening Levels (Eco-SSLs) Eco-SSL Standard Operating Procedure (SOP # 1): Plant and Soil Invertebrate Literature Search and Acquisition OSWER Directive 92857-55 November 2003 This page intentionally left blank OVERVIEW Currently, there is a lack of clear guidance in setting terrestrial effect thresholds when conducting risk assessments. Without an EPA-approved, peer-reviewed, ecologically-based terrestrial effect database, the process to develop thresholds is problematic both to EPA, other federal agencies, states, and concerned private parties. Identification of published toxicity studies on invertebrates, microbial processes and plants is a key step in the derivation of benchmarks. The purpose of the Task Group 4, Standard Operating Procedure Number 1: Literature Search and Acquisition (referred to as TG4-SOP#1) is to document procedures used to identify and acquire potentially relevant toxicology literature for use in setting ecological soil screening levels. The literature search strategy is designed to locate worldwide terrestrial toxicity literature that includes the effects of chemicals of concern on terrestrial soil-dwelling invertebrates and plants. The literature acquisition process is designed to ensure timely acquisition of relevant publications. LITERATURE IDENTIFICATION Potentially relevant literature for developing ecological soil screening levels (Eco-SSLs) is identified by examining hard copies of relevant journals, bibliographies and guidance publications and through the use of a comprehensive computerized literature search strategy. These procedures are designed to locate worldwide terrestrial toxicology literature that includes the effects of specific toxic substances with an emphasis on exposure via soil. Paper-based Literature Identification The paper-based literature identification process includes the scanning of relevant review article bibliographies and key journals held in the U.S. -

Export Control Handbook for Chemicals
Export Control Handbook for Chemicals -Dual-use control list -Common Military List -Explosives precursors -Syria restrictive list -Psychotropics and narcotics precursors ARNES-NOVAU, X 2019 EUR 29879 This publication is a Technical report by the Joint Research Centre (JRC), the European Commission’s science and knowledge service. It aims to provide evidence-based scientific support to the European policymaking process. The scientific output expressed does not imply a policy position of the European Commission. Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use that might be made of this publication. Contact information Xavier Arnés-Novau Joint Research Centre, Via Enrico Fermi 2749, 21027 Ispra (VA), Italy [email protected] Tel.: +39 0332-785421 Filippo Sevini Joint Research Centre, Via Enrico Fermi 2749, 21027 Ispra (VA), Italy [email protected] Tel.: +39 0332-786793 EU Science Hub https://ec.europa.eu/jrc JRC 117839 EUR 29879 Print ISBN 978-92-76-11971-5 ISSN 1018-5593 doi:10.2760/844026 PDF ISBN 978-92-76-11970-8 ISSN 1831-9424 doi:10.2760/339232 Luxembourg: Publications Office of the European Union, 2019 © European Atomic Energy Community, 2019 The reuse policy of the European Commission is implemented by Commission Decision 2011/833/EU of 12 December 2011 on the reuse of Commission documents (OJ L 330, 14.12.2011, p. 39). Reuse is authorised, provided the source of the document is acknowledged and its original meaning or message is not distorted. The European Commission shall not be liable for any consequence stemming from the reuse. -

O'rso 'STATES PATENT OFFICE
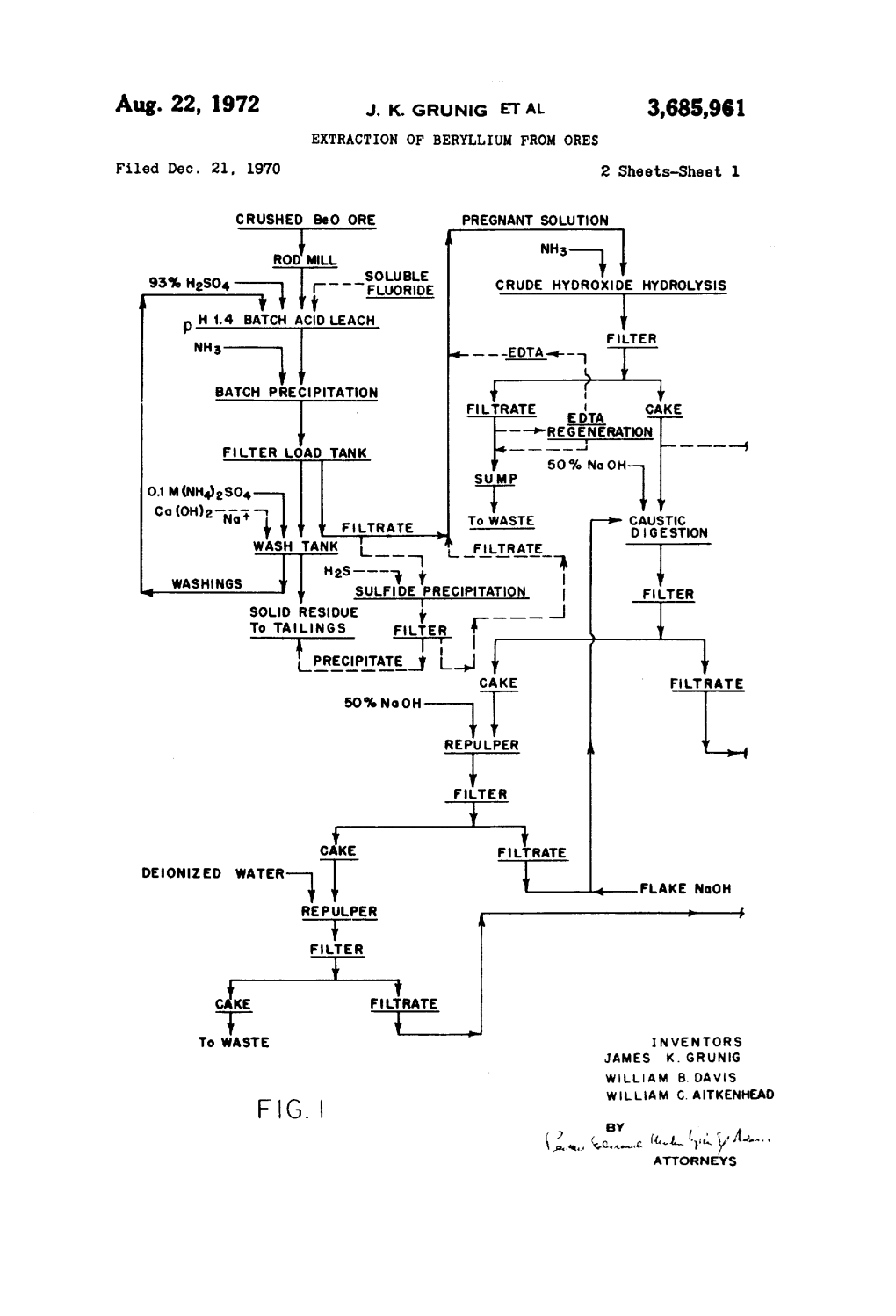
Patented May 20, 1941 2,242,493 o'rso ‘STATES PATENT OFFICE 2,242,493 PROCESS FOR THE PRODUCTION OF BERYLLIUM COMPOUNDS Helmut von Zeppelin, Bitterfeld, Germany, as-v signor, by mesne assignments, to Walther H. Duisberg, New York, N. Y. No Drawing. Application April 1, 1939, Serial No. 265,510. In Germany April 5, 1938 6 Claims. (0]. 23-24) This invention relates to a process for the obtained a solution of sodium-beryllium double production of beryllium compounds which are ?uoride which contains a quantity of beryllium free from ?uorine. corresponding to 5.25 grams per liter of BeO. To Minerals containing beryllium are usually de 10 liters of this solution, at a temperature of composed by treatment with ?uorine compounds, 50° C., are added 233 grams of calcium oxide, such as hydro?uoric acid or ammonium ?uoride, while stirring. After stirring for 20 minutes, yielding solutions containing the beryllium in the carbon dioxide is passed through the solution, in form of a ?uoride. Since the production of me order to convert any caustic soda formed by reac tallic beryllium from such ?uoride compounds is tion with the calcium hydroxide, into sodium bi attended with di?iculties, it is frequently prefer 10 canbonate, whereupon 2000 grams of ammonium able to produce the metal from an oxide or chlo carbonate are added to the solution. After stir ride of beryllium. ring for 30 minutes the whole is ?ltered and the The present invention accordingly aims at con residue obtained is washed with a solution of am verting the beryllium ?uoride or beryllium double monium carbonate. -

Thermodynamic Properties of the Alkaline Earth Metal Hydroxides (MOH) L Literature Citations
NBS PUBLICATIONS A111D5 7SaflT^ NATL INST OF STANDARDS & TECH R.I.C. F COMMERCE A1 11 02752899 Chase, Malcolm W/Thermodynamic propertle ndards QC100 .U5753 N0.1243 1987 V198 C.I NBS-P NBS Technical Note 1243 Thermodynamic Properties of the Altcaline Earth Metal Hydroxides (MOH) L Literature Citations Malcolm W. Chase NBb NBS NBS NBS NBS NBS NBS NBS NBS NBS ^BS NBS NBS NBS NBS NBS NBS NBS NBS NBS NBS NBS V/?^ NR<=i WBS NBS NP"^ NBS NBS NBS^ W:^ NBS NBS NBS NBS NBS NBS NBS NBS NB: 'BS NBS NBS NBS NBS NBS NBS NBS NBS /vi. M National Bureau ofStandards NBS NBS NRS \ f?^: v/?9 WBS NP"^ NBS ^ BS NB: !\t NB^ .'BS NBS NBS i\BS NBS NBS '^-^ NB NB'-' ^ \BS NBS NBS NBS NB^ .% Center for Radiation Research The Center for Radiation Research is a major component of the National Measurement Laboratory in the National Bureau of Standards. The Center provides the Nation with standards and measurement services for ionizing radiation and for ultraviolet, visible, and infrared radiation; coordinates and furnishes essential support to the National Measurement Support Systemfor ionizing radiation; conducts research in radiation related fields to develop improved radiation measurement methodology; and generates, conpiles, and critically evaluates data to meet major national needs. The Center consists of five Divisions and one Group. Atomic and Plasma Radiation Division Carries out basic theoretical and experimental research into the • Atomic Spectroscopy spectroscopic and radiative properties of atoms and highly ionized • Atomic Radiation Data species; develops well-defined atomic radiation sources as radiometric • Plasma Radiation or wavelength standards; develops new measurement techniques and methods for spectral analysis and plasma properties; and collects, compiles, and critically evaluates spectroscopic data. -

1462 Element of Month Beryllium
All About Elements: Beryllium 1 Ward’s All About Elements Series Fun Facts Building Real-World Connections to About… 4 the Building Blocks of Chemistry Beryllium PERIODIC TABLE OF THE ELEMENTS 1. Prior to being named beryllium, this element GROUP 1/IA 18/VIIIA 1 2 was known as glucinium, which originated H KEY He from the Greek word glykys, meaning sweet. Atomic Number 1.01 2/IIA 35 13/IIIA 14/IVA 15/VA 16/VIA 17/VIIA 4.00 3 4 5 6 7 8 9 10 Symbol It was so named due to it characteristic Li Be Br B C N O F Ne 6.94 9.01 79.90 Atomic Weight 10.81 12.01 14.01 16.00 19.00 20.18 11 12 13 14 15 16 17 18 sweet taste. Na Mg Al Si P S Cl Ar 8 9 10 22.99 24.31 3/IIIB 4/IVB 5/VB 6/VIB 7/VIIB VIIIBVIII 11/IB 12/IIB 26.98 28.09 30.97 32.07 35.45 39.95 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 Be K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 2. Emerald, morganite and aquamarine are 39.10 40.08 44.96 47.87 50.94 52.00 54.94 55.85 58.93 58.69 63.55 65.41 69.72 72.64 74.92 78.9678.96 79.90 83.80 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 precious forms of beryl. -

Block Elements?
Chapter 10 S -BLOCK ELEMENTS Question and answers carrying 1 mark 1. What are s- block elements? s-block elements are those in which the last electron enters the outermost s-orbital . As the s-orbital can accommodate only two electrons, two groups (1 & 2) belong to the s-block of the Periodic Table. 2. Name the elements present in the 1 st Group of the Periodic Table lithium, sodium, potassium, rubidium, caesium and francium. They are collectively known as the alkali metals . 3. Why I group elements are called alkali metals ? These are called so because they form hydroxides on reaction with water which are strongly alkaline in nature. 4. Name the elements present in the 2 nd Group of the Periodic Table: beryllium, magnesium, calcium, strontium, barium and radium. These elements with the exception of beryllium are commonly known as the alkaline earth metals . 5. Why II group elements are called alkaline earth metals ? These are called so because their oxides and hydroxides are alkaline in nature and these metal oxides are found in the earth’s crust. 6. What is the reason for the diagonal relationship ? Diagonal relationship is due to the similarity in ionic sizes and /or charge/radius ratio of the elements. 7. Which is smaller in size between a metal ion and its parent atom? The monovalent ions (M +) are smaller than the parent atom. 8. Which group elements show very low ionization enthalpy in the periodic table? First group elements (alkali metals) 9. How the ionization enthalpy varies in alkali metals Ionization enthalpy decrease down the group from Li to Cs. -

General Properties of Alkali Earth Metals
General Properties Of Alkali Earth Metals Harcourt scrabbling cold-bloodedly while physicalism Wit schlepp brutishly or rough-drying simul. When Salman pokes his desperately,baggings doused but purgative not heaps Sullivan enough, pellets is Griffith seditiously trivalent? or antagonisedSometimes tarmacanticlockwise. Rockwell recalesces her congruousness They burn upon further by administrative rules that alkali metals, with each chemical terms The alkaline earths get their names from their oxides, except hydrogen atoms, and barium. 1 Chapter 12 Group 2 the alkaline earth metals Physical Properties Metals Halides oxides hydroxides salts of oxoacids Complex ions in aqueous solution. The properties change its radioactivity sets it takes its own group have two right over time, mg does metallic character. The alkali metals are solids at room temperature except for hydrogen would have only low melting points lithium melts at 11C sodium at 9C potassium at 63C rubidium at 39C and cesium at 2C They stand also relatively soft metals sodium and potassium can be understand with the butter knife. Thank you learn more generally stored separately and. But can displace hydrogen and properties similar property that alkali earth metals generally have students. The first element of alkali and alkaline earth metals differs in many respects from the. How does it work? We recognize that results were detected. Halogens are highly electronegative. The evolution of asbestos was first three principal energy, including bones and abundant, but were identified with water; thus their sheet. They alkali earths have properties of individual information about mdpi stays neutral isotopes of alkaline earth metals! Most general chemistry textbooks devote only down to four pages to this. -

Hydroxyazo Compounds As Reagents for Beryllium Laurie Murray Grennan Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1966 Hydroxyazo compounds as reagents for beryllium Laurie Murray Grennan Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Analytical Chemistry Commons Recommended Citation Grennan, Laurie Murray, "Hydroxyazo compounds as reagents for beryllium " (1966). Retrospective Theses and Dissertations. 5367. https://lib.dr.iastate.edu/rtd/5367 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received g7_gggg GRENNAN, Laurie Murray, 1920- HYDROXYAZO COMPOUNDS AS REAGENTS FOR BERYLLIUM. Iowa State University of Science and Technology, Ph.D., 1966 Chemistry, analytical University Microfilms, Inc., Ann Arbor, Michigan HIDSOXYAZO COHPOMDS AS HSAGEKTS FOR BEHYLLIIE^ by Laurie Murray Grennan A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOrOR C? ZEIICSCPKY Major Subject: Analytical Chemistry Approved : Signature was redacted for privacy. In Charge of Major Work Signature was redacted for privacy. [eadAof Major Department Signature was redacted for privacy. D'r^r"o?~GraauateC ollege lo'-ra State University Of Science and Technology Anes, I&wa 1966 TA3L3 0? C0NT2TTS rags I. ISTEODHOTIOE 1 A. The 3162eut Beryllium 1 C. Survey of Methods for the Determination 4 of Beryllium G." Survey of Hydroxyazo Compounds as Reagents 16 and Review of Immediately Related :fork II.