Fluorinating Agents in Organic Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
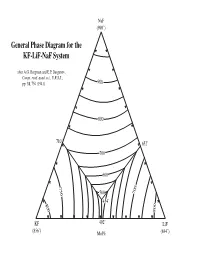
General Phase Diagram for the KF-Lif-Naf System
NaF (990˚) General Phase Diagram for the KF-LiF-NaF System after A.G. Bergman and E.P. Dergunov, Compt. rend. acad. sci., U.R.S.S., pp. 31, 754 (1941). 900 800 710˚ 652˚ 700 600 750 500 700 454˚ 800 800 KF 492˚ LiF (856˚) Mol% (844˚) Sodium Fluoride NaF 950˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 940˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 930˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 920˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 910˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 900˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 890˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 880˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 870˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 860˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 850˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 840˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 830˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 820˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 810˚ 810˚ ˚ KF 810 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 800˚ 800˚ ˚ KF 800 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 790˚ 790˚ ˚ KF 790 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 780˚ 780˚ ˚ LiF KF 780 Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 770˚ 770˚ ˚ LiF KF 770 Potassium Fluoride -

The Heat of Combustion of Beryllium in Fluorine*
JOURNAL OF RESEARCH of the National Bureau of Standards -A. Physics and Chemistry Vol. 73A, No.3, May- June 1969 The Heat of Combustion of Beryllium in Fluorine* K. L. Churney and G. T. Armstrong Institute for Materials Research, National Bureau of Standards, Washington, D.C. 20234 (February 11, 1969) An expe rimental dete rmination of the e ne rgies of combustion in Auorine of polyte traAuoroethylene film and Q.o wder and of mixtures of beryllium with polytetraAuoroethyle ne gi ves for reacti on ( 1)f).H ~.or= - 1022.22 kJ 111 0 1- 1 (- 244.32 kcal mol - I) wit h a n ove ra ll precision of 0.96 kJ 111 0 1- 1 (0. 23 kcal 111 0 1- 1 ) at the 95 pe rce nt confid ence limit s. The tota l un cert a int y is estimated not to exceed ±3.2 kJ mol- I (±0.8 kcal mol - I). The measureme nts on polytetraflu oroeth yle ne giv e for reaction (2a) and reacti on (2 b) f).H ~. o c =- 10 369. 7 and - 10392.4 Jg- I, respective ly. Overall precisions e xpressed at the 95 pe rcent confide nce Ijmits are 3.3 and 6.0 Jg- I, respective ly. Be(c)+ F,(g) = BeF2(a morphous) (1) C,F.(polym e r powd er) + 2F2(g) = 2CF.(g) (2a) C2F.(polyme r film ) + 2F2 (g) = 2CF.(g) (2b) Be2C and Be metal were observed in a small carbonaceous residue from the co mbustion of the beryll iul11 -polytetraAuoroethylene mixtures. -

Sustainable Triazine-Based Dehydro-Condensation Agents for Amide Synthesis
molecules Article Sustainable Triazine-Based Dehydro-Condensation Agents for Amide Synthesis Roberto Sole 1 , Vanessa Gatto 2 , Silvia Conca 1 , Noemi Bardella 1, Andrea Morandini 1 and Valentina Beghetto 1,2,* 1 Dipartimento di Scienze Molecolari e Nanosistemi, Università Ca’ Foscari di Venezia, Via Torino 155, 30172 Venezia, Italy; [email protected] (R.S.); [email protected] (S.C.); [email protected] (N.B.); [email protected] (A.M.) 2 Crossing srl, Viale della Repubblica 193/b, 31100 Treviso, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-041-2348928 Abstract: Conventional methods employed today for the synthesis of amides often lack of economic and environmental sustainability. Triazine-derived quaternary ammonium salts, e.g., 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMTMM(Cl)), emerged as promising dehydro-condensation agents for amide synthesis, although suffering of limited stability and high costs. In the present work, a simple protocol for the synthesis of amides mediated by 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) and a tert-amine has been described and data are compared to DMTMM(Cl) and other CDMT-derived quaternary ammonium salts (DMT-Ams(X), X: − − Cl or ClO4 ). Different tert-amines (Ams) were tested for the synthesis of various DMT-Ams(Cl), but only DMTMM(Cl) could be isolated and employed for dehydro-condensation reactions, while all CDMT/tert-amine systems tested were efficient as dehydro-condensation agents. Interestingly, in best reaction conditions, CDMT and 1,4-dimethylpiperazine gave N-phenethyl benzamide in 93% yield in 15 min, with up to half the amount of tert-amine consumption. -

"Fluorine Compounds, Organic," In: Ullmann's Encyclopedia Of
Article No : a11_349 Fluorine Compounds, Organic GU¨ NTER SIEGEMUND, Hoechst Aktiengesellschaft, Frankfurt, Federal Republic of Germany WERNER SCHWERTFEGER, Hoechst Aktiengesellschaft, Frankfurt, Federal Republic of Germany ANDREW FEIRING, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States BRUCE SMART, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States FRED BEHR, Minnesota Mining and Manufacturing Company, St. Paul, Minnesota, United States HERWARD VOGEL, Minnesota Mining and Manufacturing Company, St. Paul, Minnesota, United States BLAINE MCKUSICK, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States 1. Introduction....................... 444 8. Fluorinated Carboxylic Acids and 2. Production Processes ................ 445 Fluorinated Alkanesulfonic Acids ...... 470 2.1. Substitution of Hydrogen............. 445 8.1. Fluorinated Carboxylic Acids ......... 470 2.2. Halogen – Fluorine Exchange ......... 446 8.1.1. Fluorinated Acetic Acids .............. 470 2.3. Synthesis from Fluorinated Synthons ... 447 8.1.2. Long-Chain Perfluorocarboxylic Acids .... 470 2.4. Addition of Hydrogen Fluoride to 8.1.3. Fluorinated Dicarboxylic Acids ......... 472 Unsaturated Bonds ................. 447 8.1.4. Tetrafluoroethylene – Perfluorovinyl Ether 2.5. Miscellaneous Methods .............. 447 Copolymers with Carboxylic Acid Groups . 472 2.6. Purification and Analysis ............. 447 8.2. Fluorinated Alkanesulfonic Acids ...... 472 3. Fluorinated Alkanes................. 448 8.2.1. Perfluoroalkanesulfonic Acids -

United States Patent Office Patented July 1, 1969
3,453,337 United States Patent Office Patented July 1, 1969 1. 2 3,453,337 FLUORINATION OF HALOGENATED The presence in the reaction mixture of the two fluo ORGANIC COMPOUNDS rides, or the complex fluoride enables better yields of Royston Henry Bennett and David Walter Cottrell, Ayon highly fluorinated products to be obtained under less mouth, England, assignors to Imperial Smelting Cor severe reaction condions, markedly increases the amount poration (N.S.C.) Limited, London, England, a British of fluorination reagent reacted under otherwise similar company conditions and enables the fluorination reaction to be No brawing. Filed Feb. 19, 1965, Ser. No. 434,128 carried out (for the same yield of product) at a lower Claims priority, application Great Britain, Feb. 26, 1964, temperature with the consequent use of less costly ma 7,932/64 terials and techniques of reactor construction. The pres Int, C. C07c 25/04 10 ence of the fluorides enables the vapor phase reaction U.S. C. 260-650 2 Claims to be carried out (for the same yields) at lower pressure This invention relates to the fluorination of organic than the pressure involved in the reactions using only halogen compounds and more especially to a process the alkali metal fluorides as proposed hitherto. The fur for the production of highly fluorinated aromatic com ther possibility of using a continuous flow apparatus such pounds by the replacement of higher halogen atoms in 15 as a fluidised reactor will be apparent to those familiar halogeno-aromatic compounds by fluorine atoms. with the art. The presence of the two fluorides or com Aromatic halogenocarbons containing carbon and halo plex fluoride enables a lower temperature to be em gen atoms only can be reacted with alkali fluorides ployed than was hitherto believed to be necessary, with under various conditions to give yields of halofluoro a consequent reduction in the extent of thermal degrada aromatic compounds. -

2,4,6- Or 2,6-Alkoxyphenyl Dialkylphosphine, Tetrafluoroborate, Preparation Method and Use Thereof
(19) & (11) EP 2 492 274 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 29.08.2012 Bulletin 2012/35 C07F 9/50 (2006.01) B01J 31/24 (2006.01) C07C 43/215 (2006.01) C07C 41/30 (2006.01) (2006.01) (21) Application number: 09850497.0 C07D 307/58 (22) Date of filing: 21.12.2009 (86) International application number: PCT/CN2009/001527 (87) International publication number: WO 2011/047501 (28.04.2011 Gazette 2011/17) (84) Designated Contracting States: •LV,Bo AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Zhejiang 310027 (CN) HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL • FU, Chunling PT RO SE SI SK SM TR Zhejiang 310027 (CN) (30) Priority: 22.10.2009 CN 200910154029 (74) Representative: Meyer-Dulheuer, Karl-Hermann Dr. Meyer- Dulheuer & Partner (71) Applicant: Zhejiang University Patentanwaltskanzlei Hangzhou, Zhejiang 310027 (CN) Mainzer-Landstrasse 69-71 60329 Frankfurt am Main (DE) (72) Inventors: • MA, Shengming Zhejiang 310027 (CN) (54) 2,4,6- OR 2,6-ALKOXYPHENYL DIALKYLPHOSPHINE, TETRAFLUOROBORATE, PREPARATION METHOD AND USE THEREOF (57) The current invention relates to the structure, current invention uses only one step to synthesize dialkyl synthesis of dialkyl(2,4,6- or 2,6-alkoxyphenyl)phos- (2,4,6- or 2,6-alkoxyphenyl)phosphine and its tetrafluor- phine or its tetrafluoroborate, as well as its applications oborate is stable in the air. Compared with known syn- in the palladium catalyzed carbon-chlorine bond activa- thetic routes of ligands used in activating carbon- chlorine tion for Suzuki coupling reactions and carbon-nitrogen bonds, the method of current invention is short, easy to bond formation reactions. -

Supporting Information Degradation
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is © The Royal Society of Chemistry 2021 Supporting Information Degradation Pathways of a highly active iron(III) tetra-NHC Epoxidation Catalyst Florian Dyckhoff, Jonas F. Schlagintweit, Marco A. Bernd, Christian H. G. Jakob, Tim P. Schlachta, Benjamin J. Hofmann, Robert M. Reich and Fritz E. Kühn* Molecular Catalysis, Catalysis Research Center and Department of Chemistry, Technische Universität München, Lichtenbergstrasse 4, D-85748 Garching bei München, Germany. [*] Corresponding author: Prof. Dr. Fritz E. Kühn, E-Mail: [email protected] Table of Contents 1. Effects of bases on 2, 2-tBuCN and 2-PhCN – Catalysis and UV/vis Spectroscopy 2 2. 1H-NMR Spectroscopic Studies 5 3. Synthesis and Characterization of compound 2-d8 7 4. ESI-MS Decomposition Studies 27 5. DFT Calculations 31 6. References 34 1 1. Effects of bases on 2, 2-tBuCN and 2-PhCN – Catalysis and UV/vis Spectroscopy All batch and time-dependent reactions were conducted in a cryostat (Julabo FP-50) with a total reaction volume of 2.0 mL. The catalyst (0.05 mol%, 0.067 µmol) was added from a preformed stock solution (4.0 mg/mL in the respective nitrile, i.e. acetonitrile, tert-butylnitrile or benzonitrile) according to the appropriate stoichiometry to a solution of cis-cyclooctene (100 mol%, 134.5 µmol), the respective additive (0.5 mol%, 0.67 µmol) and H2O2 (150 mol%, 202 µmol, 50% solution in H2O) in the appropriate solvent. The reaction was initiated upon addition of H2O2. The reaction was aborted by adding electrolytically precipitated activated MnO2 as a H2O2 decomposition agent. -

Calcium Fluoride Scale Prevention
Tech Manual Excerpt Water Chemistry and Pretreatment Calcium Fluoride Scale Prevention Calcium Fluoride Fluoride levels in the feedwater of as low as 0.1 mg/L can create a scaling potential if Scale Prevention the calcium concentration is high. The calculation of the scaling potential is analogous to the procedure described in Calcium Sulfate Scale Prevention (Form No. 45-D01553-en) for CaSO4. Calculation 1. Calculate the ionic strength of the concentrate stream (Ic) following the procedure described in Scaling Calculations (Form No. 45-D01551-en): Eq. 1 2. Calculate the ion product (IPc) for CaF2 in the concentrate stream: Eq. 2 where: m 2+ 2+ ( Ca )f = M Ca in feed, mol/L m – – ( F )f = M F in feed, mol/L 3. Compare IPc for CaF2 with the solubility product (Ksp) of CaF2 at the ionic strength of the concentrate stream, Figure 2 /11/. If IPc ≥ Ksp, CaF2 scaling can occur, and adjustment is required. Adjustments for CaF2 Scale Control The adjustments discussed in Calcium Sulfate Scale Prevention (Form No. 45-D01553-en) for CaSO4 scale control apply as well for CaF2 scale control. Page 1 of 3 Form No. 45-D01556-en, Rev. 4 April 2020 Figure 1: Ksp for SrSO4 versus ionic strength /10/ Page 2 of 3 Form No. 45-D01556-en, Rev. 4 April 2020 Figure 2: Ksp for CaF2 versus ionic strength /11/ Excerpt from FilmTec™ Reverse Osmosis Membranes Technical Manual (Form No. 45-D01504-en), Chapter 2, "Water Chemistry and Pretreatment." Have a question? Contact us at: All information set forth herein is for informational purposes only. -

Environmental Impact Assessment and Due Diligence Report (DRAFT)
Environmental Impact Assessment and Due Diligence Report (DRAFT) Project Number: 47051-002 May 2015 PRC: Chemical Industry Energy Efficiency and Emissions Reduction Project Prepared by ChemChina and CHC for the Asian Development Bank. This is a version of the draft posted in May 2015 available on http://www.adb.org/Documents/RRPs/?id=47051-002-3. CURRENCY EQUIVALENTS (as of 11 May 2015) Currency unit – yuan (CNY) CNY1.00 = $ 0.1633 $1.00 = CNY 6.1254 ABBREVIATIONS ADB – Asian Development Bank AP – Affected Person API – American Petroleum Institute ASL – Above Sea Level CCPS – Center for Chemical Process Safety CEMS – Continuous Emissions Monitoring System ChemChina – China National Chemical Corporation CHC – China Haohua Company CHP – Combined Heat and Power CNY – Chinese Yuan CSEMP – Construction Site Environmental Management Plan DCS – Distributed Control System EA – Executing Agency EHS – Environment, Health and Safety EHSS – Environment, Health and Safety Specialist EHSU – Environment, Health and Safety Unit EIA – Environmental Impact Assessment EMoP – Environmental Monitoring Plan EMP – Environmental Management Plan EMS – Environmental Monitoring Station EPB – Environmental Protection Bureau FSR – Feasibility Study Report Flor – Calcium fluoride (CaF2) or “fluorite” GDP – Gross Domestic Product GHG – Greenhouse Gas GIP – Good International Practice GRM – Grievance Redress Mechanism HDP – High Density Polyethylene IA – Implementing Agency IEE – Initial Environmental Examination IT – Interim Target LIC – Loan Implementation Consultant -

Cclf3), CFC-114 (C 2Cl2f4), and CFC-115 (C2clf5
Atmos. Chem. Phys., 18, 979–1002, 2018 https://doi.org/10.5194/acp-18-979-2018 © Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Atmospheric histories and emissions of chlorofluorocarbons CFC-13 (CClF3), 6CFC-114 (C2Cl2F4), and CFC-115 (C2ClF5) Martin K. Vollmer1, Dickon Young2, Cathy M. Trudinger3, Jens Mühle4, Stephan Henne1, Matthew Rigby2, Sunyoung Park5, Shanlan Li5, Myriam Guillevic6, Blagoj Mitrevski3, Christina M. Harth4, Benjamin R. Miller7,8, Stefan Reimann1, Bo Yao9, L. Paul Steele3, Simon A. Wyss1, Chris R. Lunder10, Jgor Arduini11,12, Archie McCulloch2, Songhao Wu5, Tae Siek Rhee13, Ray H. J. Wang14, Peter K. Salameh4, Ove Hermansen10, Matthias Hill1, Ray L. Langenfelds3, Diane Ivy15, Simon O’Doherty2, Paul B. Krummel3, Michela Maione11,12, David M. Etheridge3, Lingxi Zhou16, Paul J. Fraser3, Ronald G. Prinn15, Ray F. Weiss4, and Peter G. Simmonds2 1Laboratory for Air Pollution and Environmental Technology, Empa, Swiss Federal Laboratories for Materials Science and Technology, Überlandstrasse 129, 8600 Dübendorf, Switzerland 2Atmospheric Chemistry Research Group, School of Chemistry, University of Bristol, Bristol, UK 3Climate Science Centre, CSIRO Oceans and Atmosphere, Aspendale, Victoria, Australia 4Scripps Institution of Oceanography, University of California at San Diego, La Jolla, California, USA 5Kyungpook Institute of Oceanography, Kyungpook National University, South Korea 6METAS, Federal Institute of Metrology, Lindenweg 50, Bern-Wabern, Switzerland 7Earth System Research -

Cleaning Optics
GUIDE TO CLEANING OPTICS Crystal optics are more delicate than glass and should be treated carefully if they have to be cleaned. Some materials are very delicate, and should be treated with extra care. We describe the method, but the technique comes with practice. Handle optics by the edge and use lint-free nylon gloves. Use plastic protective gloves when cleaning with solvent but check that the solvent does not attack the glove. Clean optics as little as possible and only if absolutely necessary. Cleaning may create fine scratches which you cannot see easily but which may contribute to scattering, particularly in the UV. If the optic is mounted, try never to let solvent creep into the mounting ring. Use an air jet and no solvent at all for removal of dust. Very Delicate Materials: CsI, KRS5, Germanium, Zinc Selenide, Zinc Sulphide. These materials are soft, or are inclined to take marks (sleeks) or show up marks easily. They should be washed in a solvent such as methanol or propanol for light marking. Use an environmental friendly solvent such as NuSol Rapide (a replacement for trichloroethylene which is available in the UK) or otherwise acetone, for greasy or waxy contaminants. Soak the optic and wipe while wet with cotton wool (known as absorbent cotton in the USA) dipped in the solvent and let the optic dry by evaporation or assist it with airflow as it is wiped. Delicate Materials: Calcium Fluoride, Coated Materials, Magnesium Fluoride, Other fluorides, Silicon. These should be treated as above where practical, but it is often preferable to clean them carefully with a damp tissue. -

Title Synthetic Studies on Perfluorinated Compounds
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Kyoto University Research Information Repository Synthetic Studies on Perfluorinated Compounds by Direct Title Fluorination( Dissertation_全文 ) Author(s) Okazoe, Takashi Citation Kyoto University (京都大学) Issue Date 2009-01-23 URL http://dx.doi.org/10.14989/doctor.r12290 Right Type Thesis or Dissertation Textversion author Kyoto University Synthetic Studies on Perfluorinated Compounds by Direct Fluorination Takashi Okazoe Contents Chapter I. General Introduction 1 I-1. Historical Background of Organofluorine Chemistry -Industrial Viewpoint- 2 I-1-1. Incunabula 2 I-1-2. Development with material industry 5 I-1-3. Development of fine chemicals 17 I-2. Methodology for Synthesis of Fluorochemicals 24 I-2-1. Methods used in organofluorine industry 24 I-2-2. Direct fluorination with elemental fluorine 27 I-3. Summary of This Thesis 33 I-4. References 38 Chapter II. A New Route to Perfluoro(Propyl Vinyl Ether) Monomer: Synthesis of Perfluoro(2-propoxypropionyl) Fluoride from Non-fluorinated Compounds 47 II-1. Introduction 48 II-2. Results and Discussion 49 II-3. Conclusions 55 II-4. Experimental 56 II-5. References 60 i Chapter III. A New Route to Perfluorinated Vinyl Ether Monomers: Synthesis of Perfluoro(alkoxyalkanoyl) Fluorides from Non-fluorinated Substrates 63 III-1. Introduction 64 III-2. Results and Discussion 65 III-2-1. Synthesis of PPVE precursors 65 III-2-2. Synthesis of perfluoro(alkoxyalkanoyl) fluorides via perfluorinated mixed esters 69 III-3. Conclusions 75 III-4. Experimental 77 III-5. References 81 Chapter IV. Synthesis of Perfluorinated Carboxylic Acid Membrane Monomers by Liquid-phase Direct Fluorination 83 IV-1.