Paravertebral Block: Anatomy and Relevant Safety Issues Alberto E Ardon1, Justin Lee2, Carlo D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ligaments of the Costovertebral Joints Including Biomechanics, Innervations, and Clinical Applications: a Comprehensive Review W
Open Access Review Article DOI: 10.7759/cureus.874 Ligaments of the Costovertebral Joints including Biomechanics, Innervations, and Clinical Applications: A Comprehensive Review with Application to Approaches to the Thoracic Spine Erfanul Saker 1 , Rachel A. Graham 2 , Renee Nicholas 3 , Anthony V. D’Antoni 2 , Marios Loukas 1 , Rod J. Oskouian 4 , R. Shane Tubbs 5 1. Department of Anatomical Sciences, St. George's University School of Medicine, Grenada, West Indies 2. Department of Anatomy, The Sophie Davis School of Biomedical Education 3. Department of Physical Therapy, Samford University 4. Neurosurgery, Complex Spine, Swedish Neuroscience Institute 5. Neurosurgery, Seattle Science Foundation Corresponding author: Erfanul Saker, [email protected] Abstract Few studies have examined the costovertebral joint and its ligaments in detail. Therefore, the following review was performed to better elucidate their anatomy, function and involvement in pathology. Standard search engines were used to find studies concerning the costovertebral joints and ligaments. These often- overlooked ligaments of the body serve important functions in maintaining appropriate alignment between the ribs and spine. With an increasing interest in minimally invasive approaches to the thoracic spine and an improved understanding of the function and innervation of these ligaments, surgeons and clinicians should have a good working knowledge of these structures. Categories: Neurosurgery, Orthopedics, Rheumatology Keywords: costovertebral joint, spine, anatomy, thoracic Introduction And Background The costovertebral joint ligaments are relatively unknown and frequently overlooked anatomical structures [1]. Although small and short in size, they are abundant, comprising 108 costovertebral ligaments in the normal human thoracic spine, and they are essential to its stability and function [2-3]. -

Student Workbook Answer Pages Italicized Page Numbers After the Answers Indicate Where the Informa- Matching 5) Deep Fascia Tion Can Be Found in Trail Guide
Student Workbook Answer Pages Italicized page numbers after the answers indicate where the informa- Matching 5) deep fascia tion can be found in Trail Guide. 1) N adipose—p. 17 6) adipose (fatty) tissue 2) F aponeurosis—p. 13 7) superficial fascia 3) D artery—p. 16 8) skin 4) H bone—p. 10 9) deep fascia Introduction 5) E bursa—p. 16 Tour Guide Tips #1, p. 1 6) B fascia—p. 14 1) bony landmarks—p. 2 7) G ligament—p. 13 2) Even though the topography, 8) I lymph node—p. 17 Navigating shape and proportion are unique, 9) A muscle—p. 11 Regions of the Body, p. 6 the body’s composition and struc- 10) J nerve—p. 17 1) pectoral tures are virtually identical on all 11) K retinaculum—p. 15 2) axillary individuals.—p. 2 12) L skin—p. 10 3) brachial 3) To examine or explore by touch- 13) M tendon—p. 13 4) cubital ing (an organ or area of the body), 14) C vein—p. 16 5) abdominal usually as a diagnostic aid—p. 4 6) inguinal 4) locating, aware, assessing—p. 4 Exploring Textures #1, p. 3 7) pubic 5) directs movement, depth.—p. 4 1) epidermis 8) femoral 6) • read the information 2) dermis 9) facial • visualize what you are trying 3) arrector pili muscle 10) mandibular to access 4) sweat gland 11) supraclavicular • verbalize to your partner what 5) hair follicle 12) antecubital you feel 6) blood vessels 13) patellar • locate the structure first 7) muscle fibers 14) crural on yourself 8) endomysium 15) cranial • read the text aloud 9) perimysium 16) cervical • be patient—p. -

Different Approaches to Ultrasound-Guided
REVIEW ArtICLE David S. Warner, M.D., Editor Different Approaches to Ultrasound-guided Thoracic Paravertebral Block An Illustrated Review Downloaded from http://pubs.asahq.org/anesthesiology/article-pdf/123/2/459/268052/20150800_0-00033.pdf by guest on 24 September 2021 Annelot C. Krediet, M.D., Nizar Moayeri, M.D., Ph.D., Geert-Jan van Geffen, M.D., Ph.D., Jörgen Bruhn, M.D., Ph.D., Steven Renes, M.D., Ph.D., Paul E. Bigeleisen, M.D., Gerbrand J. Groen, M.D., Ph.D. This article has been selected for the ANESTHESIOLOGY CME Program. Learning objectives and disclosure and ordering information can be found in the CME section at the front of this issue. ABSTRACT Given the fast development and increasing clinical relevance of ultrasound guidance for thoracic paravertebral blockade, this review article strives (1) to provide comprehensive information on thoracic paravertebral space anatomy, tailored to the needs of a regional anes- thesia practitioner, (2) to interpret ultrasound images of the thoracic paravertebral space using cross-sectional anatomical images that are matched in location and plane, and (3) to briefly describe and discuss different ultrasound-guided approaches to thoracic paravertebral blockade. To illustrate the pertinent anatomy, high-resolution photographs of anatomical cross-sections are used. By using voxel anat- omy, it is possible to visualize the needle pathway of different approaches in the same human specimen. This offers a unique presentation of this complex anatomical region and is inherently more realistic than anatomical drawings. (ANESTHESIOLOGY 2015; 123:459-74) HORACIC paravertebral (TPV) nerve block produces Different landmark-based techniques exist, with some Tipsilateral, segmental, somatic, and sympathetic nerve techniques using electrostimulation guidance or pressure mea- blockade in contiguous thoracic dermatomes. -

Costotransverse Articulation Injections for Treatment of Posterior Shoulder Girdle Pain Katie Gollotto, DO, Michael M. Weinik, D
Costotransverse Articulation Injections for Treatment of Posterior Shoulder Girdle Pain Katie Gollotto, DO, Michael M. Weinik, DO Department of Physical Medicine & Rehabilitation Temple University Hospital, Philadelphia, PA ABSTRACT DISCUSSION/CONCLUSION Superior costotransverse ligament Figure 1. Picture This is a patient with a medical history of CNS glioma, neurofibromatosis and thoracic The costotransverse articulation is a synovial joint attaching the tubercle of the first ten ribs to the representation scoliosis requiring fusion of C7 and T1, who presented with chronic right posterior shoulder transverse process of the corresponding vertebrae. Ribs eleven and twelve have only a costovertebral of the complex girdle and interscapular pain following a traction injury at work. On examination, she had articulation. The joint is innervated by ventral rami of the corresponding spinal nerve. The joint has a thin costotransverse tenderness over the right medial scapular border, T2 to T7 costotransverse articulations and and relatively weak articular capsule with an associated synovial cavity. There are three ligaments, which articulations. Note paraspinal musculature. There were also 3+/5 strength deficits noted in the right biceps and in synergy with the fibrous capsule, limit the degree of movement of the ribs (1). the small diameter flexor digitorum indices and bilateral triceps. Imaging revealed cervical spine degenerative disc of the aperture The superior costotransverse ligament is divided into anterior and posterior segments and attaches the rib disease and dextroscoliosis of the thoracic spine. Shoulder radiographs were negative for any through which the to the transverse process of the segment above it. This ligament creates an aperture in conjunction with the pathology. -

Evaluation and Validation of Thorax Model Responses: a Hierarchical Approach to Achieve High Biofidelity for Thoracic Musculoskeletal System
fbioe-09-712656 July 12, 2021 Time: 17:47 # 1 ORIGINAL RESEARCH published: 16 July 2021 doi: 10.3389/fbioe.2021.712656 Evaluation and Validation of Thorax Model Responses: A Hierarchical Approach to Achieve High Biofidelity for Thoracic Musculoskeletal System Wei Zeng, Sayak Mukherjee, Adrian Caudillo, Jason Forman and Matthew B. Panzer* Center for Applied Biomechanics, University of Virginia, Charlottesville, VA, United States As one of the most frequently occurring injuries, thoracic trauma is a significant public health burden occurring in road traffic crashes, sports accidents, and military events. The biomechanics of the human thorax under impact loading can be investigated by computational finite element (FE) models, which are capable of predicting complex thoracic responses and injury outcomes quantitatively. One of the key challenges for developing a biofidelic FE model involves model evaluation and validation. In this work, Edited by: the biofidelity of a mid-sized male thorax model has been evaluated and enhanced Alexandros E. Tsouknidas, University of Western Macedonia, by a multi-level, hierarchical strategy of validation, focusing on injury characteristics, Greece and model improvement of the thoracic musculoskeletal system. At the component Reviewed by: level, the biomechanical responses of several major thoracic load-bearing structures Erin Mannen, Boise State University, United States were validated against different relevant experimental cases in the literature, including Diane Wagner, the thoracic intervertebral joints, costovertebral joints, clavicle, sternum, and costal Indiana University-Purdue University cartilages. As an example, the thoracic spine was improved by accurate representation Indianapolis, United States of the components, material properties, and ligament failure features at tissue level *Correspondence: Matthew B. -

Mechanical Role of the Spine, Ribcage and Interabdominal Pressure in The
DEVELOPMENT OF A NON-FUSION SCOLIOSIS CORRECTION DEVICE NUMERICAL MODELLING OF SCOLIOSIS CORRECTION This project, “A non-fusion scoliosis correction device”, was supported by a grant from the Dutch Technology Foundation (STW), applied science division of NWO and the Technology Program of the ministry of economic affairs (project number 07618). The printing of this thesis was financially supported by: Stichting Technologische Wetenschappen (STW) Samenstelling promotiecommissie: Voorzittert en secretaris: Prof. dr. F. Eising Universiteit Twente Promotoren: Prof. dr. ir. G.J. Verkerke Universiteit Twente Prof. dr. A.G. Veldhuizen Universitair Medisch Centrum Groningen Assistent promotor: Dr. ir. J.J. Homminga Universiteit Twente Leden: Prof. dr. ir. N.J.J. Verdonschot Universiteit Twente Prof. dr. ir. A. De Boer Universiteit Twente Prof. dr. K. Ito Technische Universiteit Eindhoven Prof. dr. J.H. van Dieën Vrije Universiteit Amsterdam Prof. dr. ir. N.M. Maurits Universitair Medisch Centrum Groningen Paranimfen: Tjitske Boonstra Evelien Platvoet Printed by: Ipskamp Drukkers BV, Enschede ISBN: 978-90-365-3229-7 Copyright © 2011 by G.J.M. Meijer, Enschede, The Netherlands. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording or any information storage or retrieval system, without permission in writing from the author. DEVELOPMENT OF A NON-FUSION SCOLIOSIS CORRECTION DEVICE NUMERICAL MODELLING OF SCOLIOSIS CORRECTION PROEFSCHRIFT ter verkrijging van de graad van doctor aan de Universiteit Twente, op gezag van de rector magnificus, prof. dr. H. Brinksma, volgens besluit van het College voor Promoties in het openbaar te verdedigen op vrijdag 14 oktober 2011 om 12.45 uur door Gerarda Johanna Maria Meijer geboren op 6 december 1978 te Oldenzaal Dit proefschrift is goedgekeurd door: Prof. -

Intercostal Spaces • Eleven (11) Intercostal Spaces on Each Side • Last Two Spaces Are Open in Front
Thoracic Wall Coverings •Skin– Thin anteriorly & thick posteriorly, variable hair distribution • Superficial Fascia – More dense posteriorly • Deep Fascia – Thin , ill defined for free movement of chest for breathing • Extrinsic muscles –Upper limb , Back, Abdomen & Head & Neck Supraclavicular nerves ------ -·--------T2 - - --------·----3 - - ....- (i;:· - - - - --~ -- -- :' -,- .... :---- ; 5 -- - -- -------- -- - ---------6 -- - -----7 --- 8 Lateral - --- --- cutaneous --- -.. _ 9 ~ branches of --- --- thoracic nerves 10 Go'.)~ ------- Lateral cutaneous ........ ... .... _ .... _ branches of twelfth Lateral femoral cutaneous nerve t---- - -++- Femoral branch of genitofemoral nerve Intercostal Spaces • Eleven (11) intercostal spaces on each side • Last two spaces are open in front Features of Space • Each directed downward & forward • Narrow towards vertebral column & broad towards sternum, widest at costo-chondral junction • Posterior part is inter-osseous while ant part is inter- cartilaginous Contents – Intercostal muscles , vessels & nerves Intercostal Spaces Typical I/C space Spaces b/w typical ribs & transversed by nerves & vessels & confined to thoracic wall Boundaries of a typical I/c space – 3rd to 6th • Above – Sharp lower margin of upper rib & its cartilage •Below– Blunt upper margin of lower rib & its cartilage • In front – Lateral border of sternum b/w costal notches •Behind– Body of corresponding thoracic vertebra Intercostal muscles Arranged in three sheets from outside inward • External Intercostal • Internal Intercostal -

Sacrum: Part of Both Appendicular and Axial Skeleton
Sacrum: part of both appendicular and axial skeleton - Narrow caudal portion is sacral apex, broad superior surface base - Sacral promontory: prominent bulge at anterior tip of base - superior articular processes form synovial joints with last lumbar vertebra, only on S1 - Sacral canal begins between those processes. Spinal nerve roots continue into sacrum through this canal. extends length of sacrum ending at sacral hiatus - Median sacral crest: series of elevations formed by fused sacral vertebra - Sacral Cornu: laminae of 5th sacral vertebra don’t contact each other at midline - Sacral foramina: on either side of median sacral crest - Ala: fused transverse processes “wing” - Auricular surface: articulates with auricular surface of innominate bone - Sacral tuberosity: ligaments attach here Coccyx: -formed by 3-5(often 4) coccygeal vertebrae fusing together by 26 -Coccygeal cornu: prominent laminae of first coccygeal vertebrae, curves to meet cornua of sacrum. Males point anteriorly, females inferiorly Thoracic Cage: -Made up of thoracic vertebrae, ribs, and sternum -Protects heart, lungs, thymus, and other structures within thoracic cavity -Site of attachment for muscles involving respiration, position of the vertebral column, and movements of the pectoral girdle and upper limbs Ribs:(12 pairs) -originate on or between thoracic vertebrae, end in wall of thoracic cavity -First 7 pairs called true ribs(vertebrosternal ribs). At anterior body wall true ribs connect to sternum by separate cartilages called costal cartilages. 7th rib attaches to junction between body of sternum and xiphoid process -Beginning with first rib, ribs gradually increase in length and radius of curvature -Ribs 8-12 called false ribs (vertebrochondral ribs)don’t attach directly to sternum. -

Anatomy I Bones & Joints 2016
ANATOMY I BONES & JOINTS 2016 - 2017 10. INTRODUCTION TO THE BONES AND JOINTS ........................................................................................................ 2 11. OSTEOLOGY OF THE SPINE. THE ATLAS AND AXIS .............................................................................................. 4 12. LIGAMENTS OF THE SPINE AND THE INTERVERTEBRAL DISC. THE THORACIC CAGE ................................... 7 13. THE SHOULDER GIRDLE ......................................................................................................................................... 11 14. THE ELBOW JOINT ................................................................................................................................................... 15 15. JOINTS AND LIGAMENTS OF THE WRIST .............................................................................................................. 17 15. JOINTS AND LIGAMENTS OF THE HAND ............................................................................................................... 19 16. THE PELVIS. THE HIP JOINT ................................................................................................................................... 20 17. THE KNEE JOINT ...................................................................................................................................................... 26 18. TIBIOPERONEAL JOINTS ........................................................................................................................................ -

Curriculum 1 – Lecture 1
Curriculum 1 Lecture1 Thoracic Paravertebral Block (TPVB): Introduction, anatomy, basics of ultrasound and sonoanatomy for TPVB Why do you need to learn all that? • TPVB is a very useful block (see table for common indications) • Ultrasound is a great tool in modern medicine and the basic principles are the same and transferable TPVB is the injection of local anesthetic (LA) into the Thoracic paravertebral space (TPVS) in order to produce an ipsilateral segmental somatic and sympathetic block. TPVS is a wedge-shaped space that lies on either side of the vertebral column, in between the heads and necks of the ribs Anatomic Boundaries of TPVS and its content and communications Boundaries: Left TPVS. Paramedian sagittal view Superior/Inferior- head and neck of the ribs Anterior -parietal pleura 1 2 Posterior –medially: lower part of TP, superior costotransverse ligament (SCTL); laterally: internal intercostal membrane (IIM) - a fibrous continuation of internal intercostal muscle. External intercostal muscles 4 and levators costarum are dorsal to IIM 3 Medial - postero-lateral aspect of the vertebra, the intervertebral disc 6 and the intervertebral foramen 5 2 Lateral- Imaginary plane through lateral edge of neck of the rib (technically it extends few cm past costo-transverse junction) 1 Contents of interest: spinal nerves, sympathetic chain Communications: 1) Rib 2) Transverse process above and below: adjacent TPVSs 3) Superior costotransverse medially and anterior epidural space, mediastinum ligament (SCTL) laterally: intercostal space -

Cook, Orthopedic Manual Therapy: an Evidence-Based Approach, 2/E © 2012 by Pearson Education, Inc., Upper Saddle River, NJ Osseous Structures
THORACIC SPINE ANATOMY The thoracic spine is unique from the cervical and lumbar spine because of the size and extent of the region and the articulations with the rib cage. The articulation with the rib cage leads to regional variations in movement patterns and function (1). The upper thoracic spine mimics the movement and to some extent, the anatomy of the cervical spine and the lower thoracic vertebra mimics the lumbar spine. Table 7.1: General Information Regarding the Thoracic Spine Region. Concept Information Bones Twelve primary vertebrae, 24 individual ribs, 1 manubrium Number of dedicated 101 joints, 20 (synovial) costotransverse articulations, 24 (synovial) Joints costovertebral articulations, 20 costochondral articulations, 24 (synovial) facet articulations, and 13 intervertebral articulations. Scapulothoracic is considered a joint of the shoulder complex Anatomical regions 3 distinct regions: upper thoracic (C7-T1 toT3-4), mid-thoracic (T3-4 to T9-10; lower thoracic (T9-10 to T12-L1) Useful landmarks T5 = Nipple level T7-8 = Epigastric level T10-11 = Umbilical level Palpation: rule of threes • T1-3 SPs are in the same plane as its own transverse processes (TP) • T4-6 SPs are halfway between its own TP and the TP of the vertebra below • T7-9 SPs are in a plane with the TP of the vertebra below • T10 SP is in the plane of the TP below • T11 SP is halfway between its own TP and the TP of the vertebra below • T12 SP is in the same plane as its own TP Opening movements Flexion (both sides), side flexion (on the side away), rotation -
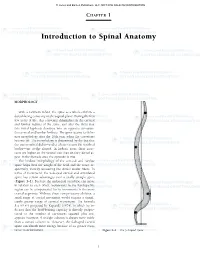
Introduction to Spinal Anatomy
55942_CH01_Vander.qxd 2/10/09 12:48 PM Page 1 © Jones and Bartlett Publishers, LLC. NOT FOR SALE OR DISTRIBUTION CHAPTER 1 Introduction to Spinal Anatomy MORPHOLOGY With a newborn infant, the spine as a whole exhibits a dorsal-facing convexity in the sagittal plane. During the first few years of life, this convexity diminishes in the cervical and lumbar regions of the spine, and after the third year this initial kyphosis develops into an opposite curvature, the cervical and lumbar lordosis. The spine attains its defin- itive morphology after the 10th year, when the curvatures become set. The morphology is determined by the fact that the intervertebral disks—and to a lesser extent the vertebral bodies—are wedge shaped. In lordotic areas, these struc- tures are higher on the ventral side than on their dorsal as- pect; in the thoracic area, the opposite is true. The lordotic morphology of the cervical and lumbar spine helps bear the weight of the head and the torso, re- spectively, thereby unloading the dorsal anular fibers. In terms of movement, the S-shaped curved and articulated spine has certain advantages over a totally straight spine (Figure 1–1). Because the individual vertebrae can move in relation to each other, movements in the lumbopelvic region can be compensated for by movements in the more cranial segments. Without these compensatory abilities, a small range of caudal movement would require a signifi- cantly greater range of cervical movement. The formula R = N2 + 1 proposed by Kapandji (1974), in which he in- dicates that the load-bearing capacity is directly propor- tional to the number of curvatures squared plus one, appears incorrect.