Odonata: Aeshnidae): a Study of Traits from Larval Development to Adults
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Foraging Behavior of Anax Junius (Odonata: Aeschnidae) and Its Potential As a Behavioral Endpoint in Pesticide Testing Sandra Kaye Brewer Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1997 The foraging behavior of Anax junius (Odonata: Aeschnidae) and its potential as a behavioral endpoint in pesticide testing Sandra Kaye Brewer Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Ecology and Evolutionary Biology Commons, Entomology Commons, Environmental Sciences Commons, Fresh Water Studies Commons, Toxicology Commons, and the Zoology Commons Recommended Citation Brewer, Sandra Kaye, "The foraging behavior of Anax junius (Odonata: Aeschnidae) and its potential as a behavioral endpoint in pesticide testing " (1997). Retrospective Theses and Dissertations. 11445. https://lib.dr.iastate.edu/rtd/11445 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. UMI MICROFILMED 1997 INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter fiice, while others may be from any type of computer printer. The quality of this reprcduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. -

Dragonflies and Damselflies in Your Garden
Natural England works for people, places and nature to conserve and enhance biodiversity, landscapes and wildlife in rural, urban, coastal and marine areas. Dragonflies and www.naturalengland.org.uk © Natural England 2007 damselflies in your garden ISBN 978-1-84754-015-7 Catalogue code NE21 Written by Caroline Daguet Designed by RR Donnelley Front cover photograph: A male southern hawker dragonfly. This species is the one most commonly seen in gardens. Steve Cham. www.naturalengland.org.uk Dragonflies and damselflies in your garden Dragonflies and damselflies are Modern dragonflies are tiny by amazing insects. They have a long comparison, but are still large and history and modern species are almost spectacular enough to capture the identical to ancestors that flew over attention of anyone walking along a prehistoric forests some 300 million river bank or enjoying a sunny years ago. Some of these ancient afternoon by the garden pond. dragonflies were giants, with This booklet will tell you about the wingspans of up to 70 cm. biology and life-cycles of dragonflies and damselflies, help you to identify some common species, and tell you how you can encourage these insects to visit your garden. Male common blue damselfly. Most damselflies hold their wings against their bodies when at rest. BDS Dragonflies and damselflies belong to Dragonflies the insect order known as Odonata, Dragonflies are usually larger than meaning ‘toothed jaws’. They are often damselflies. They are stronger fliers and referred to collectively as ‘dragonflies’, can often be found well away from but dragonflies and damselflies are two water. When at rest, they hold their distinct groups. -

Aquatic and Terrestrial Vegetation Influence
AQUATIC AND TERRESTRIAL VEGETATION INFLUENCE LACUSTRINE DRAGONFLY (ORDER ODONATA) ASSEMBLAGES AT MULTIPLE LIFE STAGES By Alysa J. Remsburg A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Zoology) at the UNIVERSITY OF WISCONSIN – MADISON 2007 i ACKNOWLEDGMENTS Reflecting on the contributions of my colleagues and friends during my graduate studies gives me a strong sense of gratitude for the community of support that I have enjoyed. The people who surround and support me deserve more thanks than I can describe here. Friends and family have supported my graduate studies by generously accommodating my tight schedule and warmly offering encouragement throughout the process. Monica Turner guided my graduate studies in numerous ways. It was her trust in my abilities and willingness to learn about a new study organism that first made this research possible. She encouraged me to pursue the research questions that most interested and inspired me, although it meant charting territory that was new to both of us. Monica served as the ideal mentor for me by requiring clear communication, modeling an efficient and balanced work ethic, providing critical reviews, and listening compassionately. This research benefited from the expertise and generosity of outstanding Wisconsin ecologists. Members of my graduate research committee, Steve Carpenter, Claudio Gratton, Tony Ives, Bobbi Peckarsky, and Joy Zedler, all offered useful suggestions and critiques on experimental design, pressing research questions, and the manuscripts. Cecile Ane provided additional statistical advice and smiles. Bill Smith, Bob DuBois, and Robert Bohanan answered (or reassured me that I should try to answer) many questions about field methods, Odonata biology, and species identification. -

Simultaneous Control of Head and Thoracic Temperature by the Green Darner Dragonfly Anax Junius (Odonata: Aeshnidae)
The Journal of Experimental Biology 198, 2373–2384 (1995) 2373 Printed in Great Britain © The Company of Biologists Limited 1995 SIMULTANEOUS CONTROL OF HEAD AND THORACIC TEMPERATURE BY THE GREEN DARNER DRAGONFLY ANAX JUNIUS (ODONATA: AESHNIDAE) MICHAEL L. MAY Department of Entomology, Cook College, New Jersey Agricultural Experiment Station, Rutgers University, New Brunswick, NJ 08903, USA Accepted 24 July 1995 Summary Anax junius is a large dragonfly that regulates thoracic during unrestrained flight in the field, Th is regulated temperature (Tth) during flight. This species, like several actively by increasing hemolymph circulation from the other intermittently endothermic insects, achieves control warm thorax at low Ta. Concurrent measurements of of Tth at least in part by increasing circulation of abdominal temperature (Tab) confirm that the abdomen is hemolymph to the abdomen at high air temperature (Ta), used as a ‘thermal window’ at Ta>30 ˚C but apparently not thus facilitating heat loss from the thorax. In this paper, I at lower Ta; thus, some additional mechanism(s) must exist demonstrate that heat transfer to the head is also under for regulation of Tth at low Ta. active control, very probably owing to temperature- sensitive alteration of hemolymph circulation. As a result, head temperature (Th) is strikingly elevated above Ta Key words: Anax junius, Anisoptera, body temperature, circulatory during endothermic warm-up and flight. Furthermore, control, dragonfly, green darner, heat exchange, thermoregulation. Introduction Numerous insects regulate Tth (most recently and The primary aim of this study is to investigate the sources comprehensively reviewed by Heinrich, 1993), among them of variation of Th, its mechanism of control and its responses the subject of this paper Anax junius (Drury) (Heinrich and to environmental temperature and internal variables in A. -

Barcode of Life Data Systems (BOLD) Versus Genbank Molecular Identification of a Dragonfly from the UAE in Comparison to the Morphological Identification
OnLine Journal of Biological Sciences Original Research Paper Barcode of Life Data Systems (BOLD) Versus GenBank Molecular Identification of a Dragonfly from the UAE in Comparison to the Morphological Identification 1Noora Almansoori, 1,2Mohamed Rizk Enan and 1Mohammad Ali Al-Deeb 1Department of Biology, United Arab Emirates University, Al-Ain, UAE 2Agricultural Research Center, Agricultural Genetic Engineering Research Institute, Giza, Egypt Article history Abstract: Dragonflies are insects in the order Odonata. They inhabit Received: 26-09-2019 freshwater ecosystems and are found in the UAE. To date, few checklists Revised: 19-11-2019 have been published for the local dragonflies and the used identification Accepted: 29-11-2019 keys are not comprehensive of Arabia. The aim of this study was to provide a molecular identification of a dragonfly based on the mitochondrial Corresponding Author: Cytochrome c Oxidase subunit I (COI) gene using the National Center for Mohammad Ali Al-Deeb, Biotechnology Information (NCBI) database and the Barcode of Life Data Department of Biology, United Systems (BOLD) in comparison with the morphology. The insect’s DNA Arab Emirates University, Al- was extracted and the PCR was performed on the target gene. The insect Ain, UAE Email: [email protected] was identified initially as Anax imperator based on the NCBI database and as Anax parthenope based on the BOLD. However, the morphological identification was in agreement with the one produced by the BOLD. The results of this study is a demonstration of how, in some cases, the DNA- based identification does not provide a conclusive species designation and that a morphology-based identification is needed. -

Identification Guide to the Australian Odonata Australian the to Guide Identification
Identification Guide to theAustralian Odonata www.environment.nsw.gov.au Identification Guide to the Australian Odonata Department of Environment, Climate Change and Water NSW Identification Guide to the Australian Odonata Department of Environment, Climate Change and Water NSW National Library of Australia Cataloguing-in-Publication data Theischinger, G. (Gunther), 1940– Identification Guide to the Australian Odonata 1. Odonata – Australia. 2. Odonata – Australia – Identification. I. Endersby I. (Ian), 1941- . II. Department of Environment and Climate Change NSW © 2009 Department of Environment, Climate Change and Water NSW Front cover: Petalura gigantea, male (photo R. Tuft) Prepared by: Gunther Theischinger, Waters and Catchments Science, Department of Environment, Climate Change and Water NSW and Ian Endersby, 56 Looker Road, Montmorency, Victoria 3094 Published by: Department of Environment, Climate Change and Water NSW 59–61 Goulburn Street Sydney PO Box A290 Sydney South 1232 Phone: (02) 9995 5000 (switchboard) Phone: 131555 (information & publication requests) Fax: (02) 9995 5999 Email: [email protected] Website: www.environment.nsw.gov.au The Department of Environment, Climate Change and Water NSW is pleased to allow this material to be reproduced in whole or in part, provided the meaning is unchanged and its source, publisher and authorship are acknowledged. ISBN 978 1 74232 475 3 DECCW 2009/730 December 2009 Printed using environmentally sustainable paper. Contents About this guide iv 1 Introduction 1 2 Systematics -

© 2016 David Paul Moskowitz ALL RIGHTS RESERVED
© 2016 David Paul Moskowitz ALL RIGHTS RESERVED THE LIFE HISTORY, BEHAVIOR AND CONSERVATION OF THE TIGER SPIKETAIL DRAGONFLY (CORDULEGASTER ERRONEA HAGEN) IN NEW JERSEY By DAVID P. MOSKOWITZ A dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey In partial fulfillment of the requirements For the degree of Doctor of Philosophy Graduate Program in Entomology Written under the direction of Dr. Michael L. May And approved by _____________________________________ _____________________________________ _____________________________________ _____________________________________ New Brunswick, New Jersey January, 2016 ABSTRACT OF THE DISSERTATION THE LIFE HISTORY, BEHAVIOR AND CONSERVATION OF THE TIGER SPIKETAIL DRAGONFLY (CORDULEGASTER ERRONEA HAGEN) IN NEW JERSEY by DAVID PAUL MOSKOWITZ Dissertation Director: Dr. Michael L. May This dissertation explores the life history and behavior of the Tiger Spiketail dragonfly (Cordulegaster erronea Hagen) and provides recommendations for the conservation of the species. Like most species in the genus Cordulegaster and the family Cordulegastridae, the Tiger Spiketail is geographically restricted, patchily distributed with its range, and a habitat specialist in habitats susceptible to disturbance. Most Cordulegastridae species are also of conservation concern and the Tiger Spiketail is no exception. However, many aspects of the life history of the Tiger Spiketail and many other Cordulegastridae are poorly understood, complicating conservation strategies. In this dissertation, I report the results of my research on the Tiger Spiketail in New Jersey. The research to investigate life history and behavior included: larval and exuvial sampling; radio- telemetry studies; marking-resighting studies; habitat analyses; observations of ovipositing females and patrolling males, and the presentation of models and insects to patrolling males. -

Discovery of an Isolated Population of Anax Longipes in Michigan (Odonata: Aeshnidae)
The Great Lakes Entomologist Volume 29 Number 3 - Fall 1996 Number 3 - Fall 1996 Article 6 October 1996 Discovery of an Isolated Population of Anax Longipes in Michigan (Odonata: Aeshnidae) Michael A. Kielb University of Michigan Mark F. O'Brien University of Michigan Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Kielb, Michael A. and O'Brien, Mark F. 1996. "Discovery of an Isolated Population of Anax Longipes in Michigan (Odonata: Aeshnidae)," The Great Lakes Entomologist, vol 29 (3) Available at: https://scholar.valpo.edu/tgle/vol29/iss3/6 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Kielb and O'Brien: Discovery of an Isolated Population of <i>Anax Longipes</i> in Mi 1996 THE GREAT LAKES ENTOMOLOGIST 161 DISCOVERY OF AN ISOLATED POPULATION OF ANAX LONGIPES IN MICHIGAN (ODONATA: AESHNIDAE) Michael A. Kielb and Mark F. O'Brien 1 ABSTRACT Anax longipes is a large aeshnid dragonfly previously unknown from Michigan. Adults and larvae were found in abundance at a series of experi mental ponds within the E.S. George Reserve in Livingston County, Michi gan. Anax longipes Hagen was previously undocumented from Michigan (Kor mondy 1958), and is rarely encountered in the Great Lakes Region (Glotzhober 1995, Kielb 1996, Tennessen 1992). The species is widely distrib uted throughout the eastern United States, from Massachusetts to Wisconsin and south to Florida, with scattered records from southern Ontario, Canada (Dunkle 1989). -

Dragonflies at All, Though
Odonatologica 5 (I): 1■ 10 March I, 1976 PROCEEDINGS OF THE THIRD INTERNATIONAL SYMPOSIUM OF ODONATOLOGY Lancaster, July 14-18, 1975 Part I A history of odonatologyin the British Isles* R.M. Gambles Windings, Whitchurch Hill, Reading RG8 7NU, England Received and Accepted December 1, 1975 The development of odonatology in the British Isles is traced from 1634 to the present and reference is made to the contributions made by British odonatologists to the knowledge of the fauna of other regions and to odonato- in the the those of K.J. logy general. Among portraits accompanying paper, MORTON (1858-1940), H. CAMPION (1869-1932), and N. MacNEILL (1899-1969)had not been published previously. I have been asked to give a brief account of the history of odonatology in the who British Isles and of British odonatologists. There are a number here present are eminently more qualified than I am for this task. But doubtless modesty would prevent them from doing justice to their own contributionto the Science. 1 Whereas I myself, having contributed nothing, need have no such inhibitions. Probably the earliest publication in this country to dealwith dragonflies at all, though they formed only a very small part of the whole„was ’Tnsectorum Theatrum” 1634, by Dr THOMAS MOUFFET (1533-1604). He did not name but them into any dragonflies, attempted a very rough classification, dividing maxima, media and minima, roughly corresponding with the familiesAeshnidae, , and the and his Libellulidae, Zygoptera, among primitive figures we can recog- nise Libellula and It contribution to depressa Calopteryx virgo. was not a great but least our Science, it was at a beginning. -

Cumulative Index of ARGIA and Bulletin of American Odonatology
Cumulative Index of ARGIA and Bulletin of American Odonatology Compiled by Jim Johnson PDF available at http://odonata.bogfoot.net/docs/Argia-BAO_Cumulative_Index.pdf Last updated: 14 February 2021 Below are titles from all issues of ARGIA and Bulletin of American Odonatology (BAO) published to date by the Dragonfly Society of the Americas. The purpose of this listing is to facilitate the searching of authors and title keywords across all issues in both journals, and to make browsing of the titles more convenient. PDFs of ARGIA and BAO can be downloaded from https://www.dragonflysocietyamericas.org/en/publications. The most recent three years of issues for both publications are only available to current members of the Dragonfly Society of the Americas. Contact Jim Johnson at [email protected] if you find any errors. ARGIA 1 (1–4), 1989 Welcome to the Dragonfly Society of America Cook, C. 1 Society's Name Revised Cook, C. 2 DSA Receives Grant from SIO Cook, C. 2 North and Central American Catalogue of Odonata—A Proposal Donnelly, T.W. 3 US Endangered Species—A Request for Information Donnelly, T.W. 4 Odonate Collecting in the Peruvian Amazon Dunkle, S.W. 5 Collecting in Costa Rica Dunkle, S.W. 6 Research in Progress Garrison, R.W. 8 Season Summary Project Cook, C. 9 Membership List 10 Survey of Ohio Odonata Planned Glotzhober, R.C. 11 Book Review: The Dragonflies of Europe Cook, C. 12 Book Review: Dragonflies of the Florida Peninsula, Bermuda and the Bahamas Cook, C. 12 Constitution of the Dragonfly Society of America 13 Exchanges and Notices 15 General Information About the Dragonfly Society of America (DSA) Cook, C. -

Observations on Egg Parasitism of Aeshna Tuberculifera (Odonata: Aeshnidae) by Eulophidae, Trichogrammatidae and Mymaridae (Hymenoptera) in Alger County, Michigan
The Great Lakes Entomologist Volume 46 Numbers 3 & 4 - Fall/Winter 2013 Numbers 3 & Article 1 4 - Fall/Winter 2013 October 2013 Observations on Egg Parasitism of Aeshna Tuberculifera (Odonata: Aeshnidae) by Eulophidae, Trichogrammatidae and Mymaridae (Hymenoptera) in Alger County, Michigan Burton C. Cebulski Mark F. O'Brien University of Michigan Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Cebulski, Burton C. and O'Brien, Mark F. 2013. "Observations on Egg Parasitism of Aeshna Tuberculifera (Odonata: Aeshnidae) by Eulophidae, Trichogrammatidae and Mymaridae (Hymenoptera) in Alger County, Michigan," The Great Lakes Entomologist, vol 46 (2) Available at: https://scholar.valpo.edu/tgle/vol46/iss2/1 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Cebulski and O'Brien: Observations on Egg Parasitism of <i>Aeshna Tuberculifera</i> (Od 2013 THE GREAT LAKES ENTOMOLOGIST 145 Observations on Egg Parasitism of Aeshna tuberculifera (Odonata: Aeshnidae) by Eulophidae, Trichogrammatidae and Mymaridae (Hymenoptera) in Alger County, Michigan Burton C. Cebulski1 and Mark F. O'Brien2 Abstract Egg parasitoids were reared from a population of Aeshna tuberculifera (Odonata: Aeshnidae) in the Kingston Lake area of Alger County, Michigan, from 1983-2005. Leaves of Iris versicolor were repeatedly used for oviposition during the period of observation, with the result that just a few leaves were targeted by different females. -
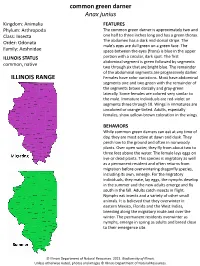
Common Green Darner Anax Junius ILLINOIS RANGE
common green darner Anax junius Kingdom: Animalia FEATURES Phylum: Arthropoda The common green darner is approximately two and Class: Insecta one half to three inches long and has a green thorax. Order: Odonata The abdomen has a dark mid-dorsal stripe. The male’s eyes are dull green on a green face. The Family: Aeshnidae space between the eyes (frons) is blue in the upper ILLINOIS STATUS portion with a circular, dark spot. The first abdominal segment is green followed by segments common, native two through six that are bright blue. The remainder of the abdominal segments are progressively darker. ILLINOIS RANGE Females have color variations. Most have abdominal segments one and two green with the remainder of the segments brown dorsally and gray-green laterally. Some females are colored very similar to the male. Immature individuals are red-violet on segments three through 10. Wings in immatures are uncolored or orange-tinted. Adults, especially females, show yellow-brown coloration in the wings. BEHAVIORS While common green darners can eat at any time of day, they are most active at dawn and dusk. They perch low to the ground and often in nonwoody plants. Over open water, they fly from about two to three feet above the water. The female lays eggs on live or dead plants. This species is migratory as well as a permanent resident and often returns from migration before overwintering dragonfly species, including its own, emerge. For the migratory individuals, they mate, lay eggs, the nymphs develop in the summer and the new adults emerge and fly south in the fall.