Discovery of an Isolated Population of Anax Longipes in Michigan (Odonata: Aeshnidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Foraging Behavior of Anax Junius (Odonata: Aeschnidae) and Its Potential As a Behavioral Endpoint in Pesticide Testing Sandra Kaye Brewer Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1997 The foraging behavior of Anax junius (Odonata: Aeschnidae) and its potential as a behavioral endpoint in pesticide testing Sandra Kaye Brewer Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Ecology and Evolutionary Biology Commons, Entomology Commons, Environmental Sciences Commons, Fresh Water Studies Commons, Toxicology Commons, and the Zoology Commons Recommended Citation Brewer, Sandra Kaye, "The foraging behavior of Anax junius (Odonata: Aeschnidae) and its potential as a behavioral endpoint in pesticide testing " (1997). Retrospective Theses and Dissertations. 11445. https://lib.dr.iastate.edu/rtd/11445 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. UMI MICROFILMED 1997 INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter fiice, while others may be from any type of computer printer. The quality of this reprcduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. -

Odonata: Aeshnidae): a Study of Traits from Larval Development to Adults
EUROPEAN JOURNAL OF ENTOMOLOGYENTOMOLOGY ISSN (online): 1802-8829 Eur. J. Entomol. 116: 269–280, 2019 http://www.eje.cz doi: 10.14411/eje.2019.031 ORIGINAL ARTICLE Biometry of the large dragonfl y Anax imperator (Odonata: Aeshnidae): A study of traits from larval development to adults MARCEAU MINOT 1, MICKAËL LE GALL2 and AURÉLIE HUSTÉ 1 1 Université de Rouen - ECODIV, Bat Blondel, Place Emile Blondel, Mont-Saint-Aignan 76821, France; e-mails: [email protected], [email protected] 2 IRSTEA, UR RIVERLY, Centre de Lyon – Villeurbanne, 69625 Villeurbanne Cedex, France; e-mail: [email protected] Key words. Odonata, Aeshnidae, Anax imperator, body length, body mass, larval rearing, sexual size dimorphism, traits Abstract. Insect larval development affects adult traits but the biometric relationships are usually poorly understood, including large odonates. In this study, measurements of morphological traits of larvae, exuviae and adults of Anax imperator were recorded. They were used to investigate the effects of early development on adult morphology. Results showed an increase in larval length during the fi nal instar and the length of its exuviae signifi cantly exceeded that of the larva. Length and body mass of teneral adults were strongly related to the length of their exuviae. Adult males were signifi cantly longer than adult females, while both had the same body mass at emergence. Length of teneral adults was negatively related to the date of emergence in both sexes. During maturation, body mass of males only increased slightly whereas that of females increased greatly. Mature specimens were also signifi cantly longer than teneral individuals. -

Barcode of Life Data Systems (BOLD) Versus Genbank Molecular Identification of a Dragonfly from the UAE in Comparison to the Morphological Identification
OnLine Journal of Biological Sciences Original Research Paper Barcode of Life Data Systems (BOLD) Versus GenBank Molecular Identification of a Dragonfly from the UAE in Comparison to the Morphological Identification 1Noora Almansoori, 1,2Mohamed Rizk Enan and 1Mohammad Ali Al-Deeb 1Department of Biology, United Arab Emirates University, Al-Ain, UAE 2Agricultural Research Center, Agricultural Genetic Engineering Research Institute, Giza, Egypt Article history Abstract: Dragonflies are insects in the order Odonata. They inhabit Received: 26-09-2019 freshwater ecosystems and are found in the UAE. To date, few checklists Revised: 19-11-2019 have been published for the local dragonflies and the used identification Accepted: 29-11-2019 keys are not comprehensive of Arabia. The aim of this study was to provide a molecular identification of a dragonfly based on the mitochondrial Corresponding Author: Cytochrome c Oxidase subunit I (COI) gene using the National Center for Mohammad Ali Al-Deeb, Biotechnology Information (NCBI) database and the Barcode of Life Data Department of Biology, United Systems (BOLD) in comparison with the morphology. The insect’s DNA Arab Emirates University, Al- was extracted and the PCR was performed on the target gene. The insect Ain, UAE Email: [email protected] was identified initially as Anax imperator based on the NCBI database and as Anax parthenope based on the BOLD. However, the morphological identification was in agreement with the one produced by the BOLD. The results of this study is a demonstration of how, in some cases, the DNA- based identification does not provide a conclusive species designation and that a morphology-based identification is needed. -

© 2016 David Paul Moskowitz ALL RIGHTS RESERVED
© 2016 David Paul Moskowitz ALL RIGHTS RESERVED THE LIFE HISTORY, BEHAVIOR AND CONSERVATION OF THE TIGER SPIKETAIL DRAGONFLY (CORDULEGASTER ERRONEA HAGEN) IN NEW JERSEY By DAVID P. MOSKOWITZ A dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey In partial fulfillment of the requirements For the degree of Doctor of Philosophy Graduate Program in Entomology Written under the direction of Dr. Michael L. May And approved by _____________________________________ _____________________________________ _____________________________________ _____________________________________ New Brunswick, New Jersey January, 2016 ABSTRACT OF THE DISSERTATION THE LIFE HISTORY, BEHAVIOR AND CONSERVATION OF THE TIGER SPIKETAIL DRAGONFLY (CORDULEGASTER ERRONEA HAGEN) IN NEW JERSEY by DAVID PAUL MOSKOWITZ Dissertation Director: Dr. Michael L. May This dissertation explores the life history and behavior of the Tiger Spiketail dragonfly (Cordulegaster erronea Hagen) and provides recommendations for the conservation of the species. Like most species in the genus Cordulegaster and the family Cordulegastridae, the Tiger Spiketail is geographically restricted, patchily distributed with its range, and a habitat specialist in habitats susceptible to disturbance. Most Cordulegastridae species are also of conservation concern and the Tiger Spiketail is no exception. However, many aspects of the life history of the Tiger Spiketail and many other Cordulegastridae are poorly understood, complicating conservation strategies. In this dissertation, I report the results of my research on the Tiger Spiketail in New Jersey. The research to investigate life history and behavior included: larval and exuvial sampling; radio- telemetry studies; marking-resighting studies; habitat analyses; observations of ovipositing females and patrolling males, and the presentation of models and insects to patrolling males. -

Dragonflies at All, Though
Odonatologica 5 (I): 1■ 10 March I, 1976 PROCEEDINGS OF THE THIRD INTERNATIONAL SYMPOSIUM OF ODONATOLOGY Lancaster, July 14-18, 1975 Part I A history of odonatologyin the British Isles* R.M. Gambles Windings, Whitchurch Hill, Reading RG8 7NU, England Received and Accepted December 1, 1975 The development of odonatology in the British Isles is traced from 1634 to the present and reference is made to the contributions made by British odonatologists to the knowledge of the fauna of other regions and to odonato- in the the those of K.J. logy general. Among portraits accompanying paper, MORTON (1858-1940), H. CAMPION (1869-1932), and N. MacNEILL (1899-1969)had not been published previously. I have been asked to give a brief account of the history of odonatology in the who British Isles and of British odonatologists. There are a number here present are eminently more qualified than I am for this task. But doubtless modesty would prevent them from doing justice to their own contributionto the Science. 1 Whereas I myself, having contributed nothing, need have no such inhibitions. Probably the earliest publication in this country to dealwith dragonflies at all, though they formed only a very small part of the whole„was ’Tnsectorum Theatrum” 1634, by Dr THOMAS MOUFFET (1533-1604). He did not name but them into any dragonflies, attempted a very rough classification, dividing maxima, media and minima, roughly corresponding with the familiesAeshnidae, , and the and his Libellulidae, Zygoptera, among primitive figures we can recog- nise Libellula and It contribution to depressa Calopteryx virgo. was not a great but least our Science, it was at a beginning. -
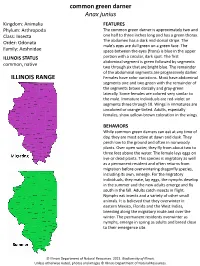
Common Green Darner Anax Junius ILLINOIS RANGE
common green darner Anax junius Kingdom: Animalia FEATURES Phylum: Arthropoda The common green darner is approximately two and Class: Insecta one half to three inches long and has a green thorax. Order: Odonata The abdomen has a dark mid-dorsal stripe. The male’s eyes are dull green on a green face. The Family: Aeshnidae space between the eyes (frons) is blue in the upper ILLINOIS STATUS portion with a circular, dark spot. The first abdominal segment is green followed by segments common, native two through six that are bright blue. The remainder of the abdominal segments are progressively darker. ILLINOIS RANGE Females have color variations. Most have abdominal segments one and two green with the remainder of the segments brown dorsally and gray-green laterally. Some females are colored very similar to the male. Immature individuals are red-violet on segments three through 10. Wings in immatures are uncolored or orange-tinted. Adults, especially females, show yellow-brown coloration in the wings. BEHAVIORS While common green darners can eat at any time of day, they are most active at dawn and dusk. They perch low to the ground and often in nonwoody plants. Over open water, they fly from about two to three feet above the water. The female lays eggs on live or dead plants. This species is migratory as well as a permanent resident and often returns from migration before overwintering dragonfly species, including its own, emerge. For the migratory individuals, they mate, lay eggs, the nymphs develop in the summer and the new adults emerge and fly south in the fall. -

International Revîëw of Poultry Science
TOME IX. 1936. No. 1/2 INTERNATIONAL REVÎËW OF POULTRY SCIENCE OFFICIAL ORGAN OF THE WORLD'S POULTRY SCIENCE ASSOCIATION ¡f73S EDITOR: Dr. B. J. C. TE HENNEPE ROTTERDAM (Holland) This Review is free fo all members of the World's Poultry Science Association. AH subscriptions should be sent to the Editor: Dr. B. J. C. te Hennepe, Rotterdam, or to the Secretary- Treasurer: Dr. G. F. Heuser, Cornell University, Ithaca, N.y., U.S.A. SUBSCRIPTIONS. $5.00 annually in advance. The personal membership of the W.P.S.A. per amounts to $5.00 year For affiliated societies „ „ $25.00 ADVERTISEMENT RATES. One page, per issue $12.00 Half page, per issue $7.00 NATIONAL POULTRY COUNCIL LAYING TRIALS REGISTER Vol. IX Containing Official Records obtained at RECOGNISED LAYING TRIALS Now on sale: Price 6d., postage paid. Copies of Vols. I-VIII are also on sale, price 6d. each, postage paid. Apply to: THE SECRETARY, National Poultry Council Avenue Chambers, 4, Vernon Place, London W. C. 1 ENGLAND Sir Edward Brown Above: Reidisminisfer for Agriculture and ßeidisbauernführer R. Walther Darré, President of the VI fh World's Poultry Congress. Left: Mr. Karl Vetter, Inspector General of the „Reichsnährstand" and President of the National Union of German Small Stock Breeders, Acting President of the VI th World's Poultry Congress. Below: Dr.Walther Kupsdi, ¡Secretary General of theVIfh World's Poultry Congress. Äf the reception of the Press in connection with the VI th World's Poultry Congress. The managing President of the Congress, Karl Vetter, explains to the Honorary President of the W.P.S.A., Sir Edward Brown, as well as to Ministerialdirektor Dr. -

Management of Arthropod Pathogen Vectors in North America: Minimizing Adverse Effects on Pollinators
Journal of Medical Entomology, 54(6), 2017, 1463–1475 Journal of Medical Entomology, 2017, 1–13 doi: 10.1093/jme/tjx146 doi: 10.1093/jme/tjx146 Advance Access Publication Date: 8 August 2017 Forum Forum Forum Management of Arthropod Pathogen Vectors in North America: Minimizing Adverse Effects on Pollinators Howard S. Ginsberg,1,2 Timothy A. Bargar,3 Michelle L. Hladik,4 and Charles Lubelczyk5 1USGS Patuxent Wildlife Research Center, University of Rhode Island, RI Field Station, Woodward Hall – PSE, Kingston, RI 02881 ([email protected]), 2Corresponding author, e-mail: [email protected], 3USGS Wetland and Aquatic Research Center, 7920 NW 71st St., Gainesville, FL 32653 ([email protected]), 4USGS California Water Science Center, 6000 J St., Placer Hall, Sacramento, CA 95819 ([email protected]), and 5Maine Medical Center Research Institute, Vector-Borne Disease Laboratory, 81 Downloaded from https://academic.oup.com/jme/article/54/6/1463/4036212 by guest on 21 July 2021 Research Dr., Scarborough, ME 04074 ([email protected]) Subject Editor: Lars Eisen Received 26 April 2017; Editorial decision 19 June 2017 Abstract Tick and mosquito management is important to public health protection. At the same time, growing concerns about declines of pollinator species raise the question of whether vector control practices might affect pollinator populations. We report the results of a task force of the North American Pollinator Protection Campaign (NAPPC) that examined potential effects of vector management practices on pollinators, and how these pro- grams could be adjusted to minimize negative effects on pollinating species. The main types of vector control practices that might affect pollinators are landscape manipulation, biocontrol, and pesticide applications. -

Arthropod Survey of 'Öpae 'Ula and Adjacent Summit Areas of the Ko
Arthropod Survey of ‘Öpae ‘ula and Adjacent Summit Areas of the Ko‘olau Mountains, O‘ahu, Hawai‘i David J. Preston , Dan A. Polhemus, Keith T. Arakaki, and Myra K. K. McShane Hawaii Biological Survey, Bishop Museum, 1525 Bernice Street Honolulu, Hawaii, 96817-2704, USA Submitted to Hawaii Department of Land And Natural Recourses Division Of Forestry And Wildlife Hawaii Natural Area Reserve System 1151 Punchbowl Street, Room 325 Honolulu, Hawaii 96813 June 2004 Contribution No.2004-010 to the Hawaii Biological Survey 1 TABLE OF CONTENTS INTRODUCTION ................................................................................................................................2 METHODS............................................................................................................................................2 COLLECTION SITES .........................................................................................................................2 Table 1: Collection sites .......................................................................................................................3 Figure 1. Sample sites...........................................................................................................................4 RESULTS..............................................................................................................................................4 Table 2: Insects and related arthropods collected from the upper Käluanui drainage, Ko’olau Mountains..............................................................................................................................................6 -

Dragonflies: Friends of Foes
The Dragonfly: Friend or Foe? By Dr. Everett D. Cashatt ISM Curator of Zoology “Mosquito hawk,” “snake doctor,” and “Devil’s darning needle” are some of the more colorful local names that refer to these large spectacular insects. Despite a widespread belief that dragonflies sting, they do not. They have no means of stinging and are completely harmless to man. In fact, life history studies indicate they are one of our most beneficial insects. Description Dragonflies and damselflies, a closely related group, belong to the order Odonata. They have large compound eyes, short, bristle-like antennae, and four elongate membranous wings. Some species have transparent, colorless or somber-hued wings; others have brilliantly colored ones ― blue, green, purple, white, or bronze. Still others may have conspicuous mottling or spotting on the wings. Not only the dragonflies’ wings, but their bodies, too, may be brightly colored. Damselflies are easily distinguished in the field by their more delicate features and the vertical position of the wings over the abdomen when at rest. Dragonflies are more robust in structure, strong fliers, and hold their wings in a horizontal position when resting. They are master aerialists, capable of swift flight, of a backward as well as a forward darting movement, and of hovering. American Rubyspot Damselfly (Hetaerina Americana) Photograph and copyright by Clark Schiffer Of approximately 5,000 species of Odonata in the world, over 360 species of dragonflies occur in the United States. There are 147 species of dragonflies that have been reported or are suspected to be present in Illinois. Present day dragonflies in the United States range in wingspread from 1 ¼” (32mm) to almost 5” (127mm). -

The Woolston Eyes Conservation Group
The Woolston Eyes Conservation Group Annual Report 2014 WOOLSTON EYES CONSERVATION GROUP ANNUAL REPORT 2014 CONTENTS Page Chairman’s Report B. Ankers 2 Weather B. Martin 3 Systematic List B. Martin 6 D. Hackett D. Spencer D. Riley D. Bowman WeBS Counts B. Martin 54 Ringing Report M. Miles 56 Recoveries and Controls M. Miles 63 Migration Watch D. Steel 71 Butterfly Report D. Hackett 75 Odonata Report Brian Baird 82 Willow Tit Report Alan Rustell 90 Heteroptera and Diptera Phil Brighton 92 Mammals D. Bowman 99 Acknowledgments D. Bowman 104 Officers of Woolston Eyes Conservation Group 105 © No part of this report may be reproduced in any way without the prior permission of the Editor. 1 CHAIRMAN’S REPORT A special welcome to all those members taking the Annual Report for the first time now that it is available online. I am sure you will find it an enjoyable and informative read. Our income comes from the sale of permits and any grants that we manage to acquire, and all monies are put back into the Reserve. As you are aware, the management of the Reserve has always been undertaken by volunteers, but specialist work is done by our contractors so this is where a lot of our income is spent. I would therefore like to thank all of you for your continued support in renewing your permits, sending in donations and, in two cases, for bequests leaving money to the Reserve in wills. You will see on the cover of our report the wonderful drawing produced free of charge to us by the highly thought of wildlife artist Colin Woolf. -

Observation of the Vagrant Emperor (Anax Ephippiger, ODONATA: AESHNIDAE) in Sri Lanka After 38 Years
Ceylon Journal of Science (Bio. Sci.) 43 (2): 83-84, 2014 DOI: http://dx.doi.org/10.4038/cjsbs.v43i2.7328 RESEARCH NOTE Observation of the Vagrant Emperor (Anax ephippiger, ODONATA: AESHNIDAE) in Sri Lanka after 38 years Nayana Wijayathilaka*, Nirodha Abayalath and Champika Bandara Department of Zoology, Faculty of Science, University of Peradeniya, Sri Lanka. Accepted November 30, 2014 ABSTRACT Anax ephippiger is a widespread dragonfly species whose range extends from Sri Lanka and India through Africa to the Mediterranean region and Europe. However, the species is previously known only from two sightings in Sri Lanka. Thirty-eight years after the last sighting of the species, the authors photographed a male A. ephippiger on 3rd of November, 2008 around 4.00 pm. Keywords: Aeshnidae, dragonfly; migratory, Odonata. INTRODUCTION The genus is represented in Sri Lanka by five species: A. ephippiger, A. guttatus, A. Sri Lanka is home to 124 species of dragonflies, of immaculifrons, A. indicus and A. tristis. Globally, which 61 (49.2%) are endemic to the island at the A. ephippiger is a widespread species and its range species or subspecies level (Bedjanič et al. 2014). extends from Sri Lanka and India through Africa Anax ephippiger, the Vagrant Emperor, first to the Mediterranean region and Europe. It has described by Burmeister in 1839, belongs to the been assessed and given the status of ‘Least family Aeshnidae (Hawkers) in the order Odonata. Concern’ at the global level by the IUCN (Subramanian, 2013). Figure 1: Sightings of Anax ephippiger in Sri Lanka. *Corresponding author’s email: [email protected] 84 Wijayathilaka et al.