Standard Operating Procedure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
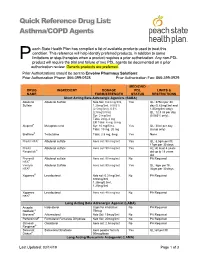
Asthma/COPD Agents
Quick Reference Drug List: Asthma/COPD Agents each State Health Plan has compiled a list of available products used to treat this condition. This reference will help identify preferred products, in addition to some P limitations or step-therapies when a product requires a prior authorization. Any non-PDL product will require the trial and failure of two PDL agents be documented on a prior authorization review. Generic products are preferred. Prior Authorizations should be sent to Envolve Pharmacy Solutions: Prior Authorization Phone: 866-399-0928 Prior Authorization Fax: 866-399-0929 MEDICAID DRUG INGREDIENT DOSAGE PDL LIMITS & NAME FORM/STRENGTH STATUS RESTRICTIONS Short Acting Beta Adrenergic Agonists (SABA) Albuterol Albuterol Sulfate Neb Sol: 0.63 mg/3ml, Yes QL: 375ml per 30 Sulfate 1.25mg/3ml, 0.083% day (0.63mg/3ml and (2.5mg/3ml), 0.5% 1/25mg/3ml only); (2.5mg/0.5ml) QL: 12.5 ml per day Syr: 2 mg/5ml (0.083% only); Tabs: 2mg, 4 mg ER Tabs: 4 mg, 8 mg Alupent® Metaproterenol Syr: 10 mg/5 mL Yes QL: 30ml per day Tabs: 10 mg, 20 mg (syrup only) Brethine® Terbutaline Tabs: 2.5 mg, 5mg Yes None ProAir HFA® Albuterol sulfate Aero sol: 90 mcg/act Yes QL: 8.5gm per fill, 17gm per 30 days ProAir Albuterol sulfate Aero sol: 90 mcg/act Yes AL: At least 4 years Respiclick® old up to 18 years old Proventil Albuterol sulfate Aero sol: 90 mcg/act No PA Required HFA® Ventolin Albuterol Sulfate Aero sol: 90 mcg/act Yes QL: 8gm per fill, HFA® 36gm per 30 days Xopenex® Levalbuterol Neb sol: 0.31mg/3ml, No PA Required 0.63mg/3ml, 1.25mg/0.5ml, 1.25mg/3ml -

Ophthalmic Adverse Effects of Nasal Decongestants on an Experimental
A RQUIVOS B RASILEIROS DE ORIGINAL ARTICLE Ophthalmic adverse effects of nasal decongestants on an experimental rat model Efeitos oftálmicos adversos de descongestionantes nasais em modelo experimental com ratos Ayse Ipek Akyuz Unsal1, Yesim Basal2, Serap Birincioglu3, Tolga Kocaturk1, Harun Cakmak1, Alparslan Unsal4, Gizem Cakiroz5, Nüket Eliyatkın6, Ozden Yukselen7, Buket Demirci5 1. Department of Ophthalmology, Medical Faculty, Adnan Menderes University, Aydin, Turkey. 2. Department of Otorhinolaringology, Medical Faculty, Adnan Menderes University, Aydin, Turkey. 3. Department of Pathology, Veterinary Faculty, Adnan Menderes University, Aydin, Turkey. 4. Department of Radiology, Medical Faculty, Adnan Menderes University, Aydin, Turkey. 5. Department of Medical Pharmacology, Medical Faculty, Adnan Menderes University, Aydin, Turkey. 6. Department of Medical Pathology, Medical Faculty, Adnan Menderes University, Aydin, Turkey. 7. Department of Pathology, Aydin State Hospital, Aydin, Turkey. ABSTRACT | Purpose: To investigate the potential effects of cause ophthalmic problems such as dry eyes, corneal edema, chronic exposure to a nasal decongestant and its excipients cataracts, retinal nerve fiber layer, and vascular damage in on ocular tissues using an experimental rat model. Methods: rats. Although these results were obtained from experimental Sixty adult male Wistar rats were randomized into six groups. animals, ophthalmologists should keep in mind the potential The first two groups were control (serum physiologic) and ophthalmic adverse effects of this medicine and/or its excipients Otrivine® groups. The remaining four groups received the and exercise caution with drugs containing xylometazoline, Otrivine excipients xylometazoline, benzalkonium chloride, ethylene diamine tetra acetic acid, benzalkonium chloride and sorbitol, and ethylene diamine tetra acetic acid. Medications sorbitol for patients with underlying ocular problems. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group R01 (Nasal preparations) Table of Contents Page INTRODUCTION 5 DISCLAIMER 7 GLOSSARY OF TERMS USED IN THIS DOCUMENT 8 ACTIVE SUBSTANCES Cyclopentamine (ATC: R01AA02) 10 Ephedrine (ATC: R01AA03) 11 Phenylephrine (ATC: R01AA04) 14 Oxymetazoline (ATC: R01AA05) 16 Tetryzoline (ATC: R01AA06) 19 Xylometazoline (ATC: R01AA07) 20 Naphazoline (ATC: R01AA08) 23 Tramazoline (ATC: R01AA09) 26 Metizoline (ATC: R01AA10) 29 Tuaminoheptane (ATC: R01AA11) 30 Fenoxazoline (ATC: R01AA12) 31 Tymazoline (ATC: R01AA13) 32 Epinephrine (ATC: R01AA14) 33 Indanazoline (ATC: R01AA15) 34 Phenylephrine (ATC: R01AB01) 35 Naphazoline (ATC: R01AB02) 37 Tetryzoline (ATC: R01AB03) 39 Ephedrine (ATC: R01AB05) 40 Xylometazoline (ATC: R01AB06) 41 Oxymetazoline (ATC: R01AB07) 45 Tuaminoheptane (ATC: R01AB08) 46 Cromoglicic Acid (ATC: R01AC01) 49 2 Levocabastine (ATC: R01AC02) 51 Azelastine (ATC: R01AC03) 53 Antazoline (ATC: R01AC04) 56 Spaglumic Acid (ATC: R01AC05) 57 Thonzylamine (ATC: R01AC06) 58 Nedocromil (ATC: R01AC07) 59 Olopatadine (ATC: R01AC08) 60 Cromoglicic Acid, Combinations (ATC: R01AC51) 61 Beclometasone (ATC: R01AD01) 62 Prednisolone (ATC: R01AD02) 66 Dexamethasone (ATC: R01AD03) 67 Flunisolide (ATC: R01AD04) 68 Budesonide (ATC: R01AD05) 69 Betamethasone (ATC: R01AD06) 72 Tixocortol (ATC: R01AD07) 73 Fluticasone (ATC: R01AD08) 74 Mometasone (ATC: R01AD09) 78 Triamcinolone (ATC: R01AD11) 82 -

Fee-For-Service Preferred Drug List
Prescription Drug Program Apple Health Medicaid: Fee-for-Service Preferred Drug List What is new in this version of the preferred drug list? Effective for dates of service on and after October 1, 2018, the Health Care Authority will make the following changes: Change Due to the implementation of the Apple Health Preferred Drug List (PDL), a PDL that applies to fee-for-service (FFS) clients as well as Apple Health managed care enrollees, the following changes have occurred: On the Fee-For-Service only Preferred Drug List • Drug classes that are currently on the Apple Health PDL have been removed. These classes and the drug statuses can be found on the Apple Health Preferred Drug List. On the Apple Health Preferred Drug List • New drug classes have been added. This means drugs not previously on the PDL have been added with preferred and nonpreferred statuses. Some drugs may also have additional prior authorization (PA) requirements. • For existing drug classes, preferred statuses may have changed. Some drugs may have additional PA requirements that did not previously require PA. October 25, 2018 Correction • Two drug classes were erroneously removed and have been added back to the list: Asthma – Leukotriene Modifiers Skeletal Muscle Relaxants (Rev. 10/24/2018) (Eff. 10/1/2018) – 1 – Apple Health Medicaid PDL Prescription Drug Program What is the preferred drug list? The Health Care Authority (the agency) has developed a list of preferred drugs within a chosen therapeutic class that are selected based on clinical evidence of safety, efficacy, and effectiveness. The drugs within a chosen therapeutic class are evaluated by the Drug Use Review Board, which makes recommendations to the agency regarding the selection of the preferred drugs. -

Study Protocol
RESEARCH PROTOCOL Project Title A multicenter, single blind, randomized controlled trial of virucidal effect of Polyvinylpyrrolidone-Iodine on SARS-CoV-2 as well as safety of its application on nasopharynx & oropharynx of COVID-19 positive patients BMRC Reg. No: 38624012021 Page-1/17 Project Title A multicenter, single blind, randomized controlled trial of virucidal effect of Polyvinylpyrrolidone- Iodine on SARS-CoV-2 as well as safety of its application on nasopharynx & oropharynx of COVID- 19 positive patients. Summary Povidone Iodine (Iodine with water soluble polymer Polyvinylpyrolidone) or PVP-I is a proven and time trusted antiseptic agent having best possible (99.99%) virucidal effect in it‟s only 0.23% concentration, against all viruses including SARS-Co, MERS-CoV; even in SARS-COV-2 due to it‟s nonspecific mode of action for virus killing and having no resistance [1,2]. Corona virus is transmitted by/via respiratory droplets or aerosol, produced from sneezing or coughing of infected persons to healthy individual through mouth and nose mainly [5, 6]. The routes of entry of coronavirus in human body are mouth, nose and eye. PVP-I products for gargling the throat and spraying or washing the nose may have a preventive effect on COVID-19 and if it is proved in this study following human trial, this will be a landmark research in COVID-19 pandemic. In line of this, PVP-I containing oro-nasal spray, proposed Bangasafe, which should be regarded as PONS (Povidone Iodine oro-nasal spray) in this protocol, has been developed and proposed to use against corona virus disease. -

Composition for Rectal Administration Combined with an Oral Alpha-Lipoic
(19) & (11) EP 2 162 125 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 31/136 (2006.01) A61K 31/01 (2006.01) 19.10.2011 Bulletin 2011/42 A61P 1/00 (2006.01) A61K 45/06 (2006.01) A61K 31/56 (2006.01) A61K 31/573 (2006.01) (2006.01) (21) Application number: 08768441.1 A61K 31/606 (22) Date of filing: 13.06.2008 (86) International application number: PCT/US2008/007401 (87) International publication number: WO 2008/156671 (24.12.2008 Gazette 2008/52) (54) Composition for rectal administration combined with an oral alpha-lipoic acid composition for the treatment of inflammatory bowel disease Zusammensetzung zur rektalen Verabreichung kombiniert mit einer oralen Zusammentzung enthaltend alpha Liponsäure zur Behandlung von entzündlicher Darmerkrankung Composition pour l’administration rectale en combinaison avec une composition orale de l’ acide alpha- lipoique pour le traitment des maladies intestinales inflammatoires (84) Designated Contracting States: (56) References cited: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR EP-B1- 0 671 168 WO-A2-02/089796 HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT US-A- 4 657 900 US-A- 5 082 651 RO SE SI SK TR US-A- 5 378 470 (30) Priority: 13.06.2007 US 934505 P • MULDER CJ. ET AL.: ’Beclomethasone 06.02.2008 US 63745 P dipropionate (3mg) versus 5- aminosalicylic acid (2g) versus the combination of both (3mg/2g)as (43) Date of publication of application: retention enemas in active ulcerative proctitis’ 17.03.2010 Bulletin 2010/11 EUR.J.GASTROENTEROL HEPATOL vol. -

Inhaled Corticosteroid (ICS) Only – Metered Dose Inhalers (MDI)
Categorisation and Formulary Choices of Inhaled Corticosteroids for Adults Inhaled Corticosteroid (ICS) only – Metered Dose Inhalers (MDI) MDI Beclometasone dipropionate Use with a compatible Fluticasone Propionate spacer device Prescribe by BRAND Clenil Modulite® Qvar®(extrafine) Qvar®(extrafine) Flixotide® Evohaler MDI Easi-Breathe Low Dose 100mcg, two puffs twice a 50 mcg, two puffs twice a day 50 mcg, two puffs twice a day 50 mcg, two puffs twice a day (BDP equivalent day (200 dose) (200 dose) (200 dose) (120 dose) ≤400mcg BDP) £4.45/month £4.72/month £4.64/month £5.44/month Medium Dose 200mcg, two puffs twice a 100 mcg, two puffs twice a day 100 mcg, two puffs twice a day 125mcg, two puffs twice a day (BDP equivalent day (200 dose) (200 dose) (200 dose) (120 dose) 400-1000mcg BDP) £9.70/month £10.33/month £10.17/month £12.50/month High Dose 250mcg, two puffs twice a 100 mcg, four puffs twice a day 100 mcg, four puffs twice a day 250mcg, two puffs twice a day (BDP equivalent day (200 dose) (200 dose) (200 dose) (120 dose) >1000mcg BDP) £9.77/month £20.66/month £20.34/month £20.00/month Simultaneous specialist OR referral recommended 250mcg four puffs, twice a day (200 dose) £19.55/month Prices correct as per Drug Tariff and DM&D December2018 1st line choices (dark green, solid border) 2nd line choices (light green, dashed border) Approved by Hertfordshire Medicines Management Committee – October 2018 Inhaled Corticosteroid (ICS) only – Dry Powder Inhalers (DPI) Beclometasone DPI dipropionate Budesonide Fluticasone Propionate Prescribe -

Topical Management of Chronic Rhinosinusitis
Open Access Advanced Treatments in ENT Disorders Review Article Topical Management of chronic rhinosinusitis - A literature review ISSN 1 2 2640-2777 Aremu Shuaib Kayode * and Tesleem Olayinka Orewole 1ENT Department, Federal Teaching Hospital, Ido-Ekiti/Afe Babalola University, Ado Ekiti, Nigeria 2Department of Anaesthesia, Federal Teaching Hospital, Ido Ekiti/Afe Babalola University, Ado Ekiti, Nigeria *Address for Correspondence: Dr. Aremu Shuaib Introduction Kayode, ENT Department, Federal Teaching Chronic rhinosinusitis (CRS) is an inlammatory condition involving nasal passages Hospital, Ido-Ekiti/Afe Babalola University, Ado Ekiti, Nigeria, Tel: +2348033583842; and the paranasal sinuses for 12 weeks or longer [1]. It can be subdivided into three Email: [email protected] types: CRS with nasal polyposis (CRS with NP), CRS without nasal polyposis (CRS Submitted: 08 April 2019 without NP), and Allergic fungal rhinosinusitis (AFRS). To diagnose CRS we require Approved: 25 April 2019 at least two of four of its cardinal signs/symptoms (nasal obstruction, mucopurulent Published: 26 April 2019 discharge, facial pain/pressure, and decreased sense of smell). In addition, direct Copyright: © 2019 Aremu SK, et al. This is visualization or imaging for objective documentation of mucosal inlammation is an open access article distributed under the required. CRS therapy is aimed to reduce its symptoms and improve quality of life as it Creative Commons Attribution License, which cannot be cured in most patients. Thus, the goals of its therapy include the following: permits unrestricted use, distribution, and reproduction in any medium, provided the 1: Control mucosal edema and inlammation of nasal and paranasal sinuses original work is properly cited 2: Maintain adequate sinus ventilation and drainage 3: Treat any infecting or colonizing micro-organisms, if present 4: Reduce the number of acute exacerbations Mucosal remodeling is the most likely underlying mechanism causing irreversible chronic sinus disease, similar to that occur in severe asthma. -

7.Inhalation Testing
Analytical Challenges in Implementing Effective QC/Stability testing of Inhalation Drug Products Wayland Rushing, Ph.D. Director, Scientific Affairs Eurofins BioPharma Product Testing www.Eurofins.com Inhalation Overview Delivery of one or more drug products to the lungs or nasal mucosa Benefits: § Directly targets the lungs § Rapid onset of drug action • Quick absorption into bloodstream § Low doses required § Fewer side effects 2 Drug/Device Combination Devices Orally Inhaled and Nasal Drug Products (OINDP) § Metered Dose Inhaler (pMDI or simply MDI) • HFA propellant Driven § Dry Powder Inhaler (DPI) • Patient driven § Nebulizer • Aerosolizes solutions for constant delivery § Nasal Spray • Liquid or powder delivery to nasal cavity 3 Regulatory Guidance FDA Guidances § Guidance for Container Closure Systems for Packaging Human Drugs and Biologics (1999) § Reviewers Guidance for Nebulizers, Metered Dose Inhalers, Spacers and Actuators (1993) § Metered Dose Inhalers (MDI) and Dry Powder Inhalers (DPI) (revised 2018) § Nasal Spray and Inhalers Solution, Suspension, and Spray Drug Products (2002) § Drug Products Packaged in Semipermeable Container Closure Systems (2002) USP Chapters § USP <5>: Inhalation and Nasal Drug Products—General Information and Product Quality Tests § USP <601>: Inhalation and Nasal Drug Products: Aerosols, Sprays, and Powders— Performance Quality Tests § USP <1602>: Spacers and Valved Holding Chambers Used with Inhalation Aerosols— Characterization Tests § USP <1664.1>: Orally Inhaled and Nasal Drug Products 4 Revise -

Nasal and Sinus Surgery After Care
ENT SURGICAL CONSULTANTS 2201 Glenwood Ave., Joliet, IL 60435 (815) 725-1191, (815) 725-1248 fax Michael G. Gartlan, MD, FAAP, FACS 1890 Silver Cross Blvd, Pavilion A, Suite 435, New Lenox, IL 60451 Rajeev H. Mehta, MD, FACS 815-717-8768 Scott W. DiVenere, MD Sung J. Chung, MD 900 W. Rte 6, Suite 960., Morris, IL 60450 (815) 941-1972 Ankit M. Patel, MD Walter G. Rooney, MD www.entsurgicalillinois.com NASAL & SINUS SURGERY (7/13) Nasal Drainage and Bleeding Immediately after surgery, you will have drainage from your nose. At first, there may be a small amount of bright red bleeding, but do not be alarmed. A small amount is normal and may continue through the first week. A gauze dressing will be place on your upper lip to absorb this drainage. It may be necessary to change this dressing several times on the day of your surgery. Any bright red bleeding that lasts more than ten minutes, orEar, is heavy, Nose &spray Throat two Diseases puffs of over-the-counter Afrin decongestant nasal spray (or generic equivalent) in each nostril every ten minutesOtolaryngology until the bleeding-Head & subsides. Neck Surgery This should only be used for active bleeding. This medication is available. Facial Plastic & Reconstructive Surgery Relax with your head elevated,Thyroid tilt your and headParathyroid forward Surgery and gently pinch your nostrils with an ice pack over the bridge of your nose. Persistent bleeding should be reportedPediatric to Otolaryngologyyour doctor immediately. Old blood, which accumulated during surgery, is dark reddish-brown and will drain for a week or more. -

BSG Plan for IBD Patients During COVID19 Pandemic V1.5 349.88 KB
British Society of Gastroenterology (BSG) advice for management of inflammatory bowel diseases during the COVID-19 pandemic Date of publication: 22nd March 2020 Introduction Inflammatory bowel disease (IBD), comprising Crohn’s disease (CD) and ulcerative colitis (UC), is a condition in which the gastrointestinal immune system responds inappropriately. It is therefore often treated with immune suppression medications to control inflammation and to prevent ‘flares’, a worsening in symptoms, which may be unpredictable. It is known that 0.8% of people in the UK (approx. 524,000 patients) currently have IBD, but only 44% have been to a clinic in the last 3 years1,2. There will be many patients who will be worried about the effect of the Coronavirus pandemic (SARS-CoV-2 or COVID-19 disease) on their IBD and vice versa. During the COVID-19 outbreak we will do everything we can to keep our IBD patients safe. The biggest risks are related not only to the infection itself, but also the emergency reorganisation of hospital and general practice services to deal with the pandemic, meaning routine IBD services will be significantly affected. A combined approach covering both primary and secondary care is therefore required to keep vulnerable IBD patients out of hospital as much as possible. Insights from Hubei, China and from Italy suggest hospital admission for non-COVID-19 illness will provide a reservoir for further spread of infection. However, alterations to the way we deliver IBD care in the UK must be balanced against the risks of undertreated, active IBD. Importantly, patients with active IBD are likely to have a higher risk of infection both in the community and during inpatient care, even in the absence of immunosupressant treatment3. -

FF/UMEC/VI) Triple Therapy Versus Tiotropium Monotherapy in Patients with COPD
www.nature.com/npjpcrm ARTICLE OPEN Single-inhaler fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) triple therapy versus tiotropium monotherapy in patients with COPD Sandeep Bansal1, Martin Anderson2, Antonio Anzueto3,4, Nicola Brown5, Chris Compton6, Thomas C. Corbridge7,8, David Erb9, Catherine Harvey5, Morrys C. Kaisermann10, Mitchell Kaye11, David A. Lipson 10,12, Neil Martin6,13, Chang-Qing Zhu5 and ✉ Alberto Papi 14 Chronic obstructive pulmonary disease (COPD) treatment guidelines do not currently include recommendations for escalation directly from monotherapy to triple therapy. This 12-week, double-blind, double-dummy study randomized 800 symptomatic moderate-to-very-severe COPD patients receiving tiotropium (TIO) for ≥3 months to once-daily fluticasone furoate/umeclidinium/ vilanterol (FF/UMEC/VI) 100/62.5/25 mcg via ELLIPTA (n = 400) or TIO 18 mcg via HandiHaler (n = 400) plus matched placebo. Study endpoints included change from baseline in trough forced expiratory volume in 1 s (FEV1) at Days 85 (primary), 28 and 84 (secondary), health status (St George’s Respiratory Questionnaire [SGRQ] and COPD Assessment Test [CAT]) and safety. FF/UMEC/VI significantly improved trough FEV1 at all timepoints (Day 85 treatment difference [95% CI] 95 mL [62–128]; P < 0.001), and significantly improved SGRQ and CAT versus TIO. Treatment safety profiles were similar. Once-daily single-inhaler FF/UMEC/VI significantly improved lung function and health status versus once-daily TIO in symptomatic moderate-to-very-severe COPD 1234567890():,; patients, with a similar safety profile. npj Primary Care Respiratory Medicine (2021) 31:29 ; https://doi.org/10.1038/s41533-021-00241-z INTRODUCTION performed to date and a lack of updated recommendations Chronic obstructive pulmonary disease (COPD) is a major cause of based on the current body of evidence.