Experiment 1: Usage and Calibrate of Lab 1/7-7-2010 3 Glassware Equipment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laboratory Glassware N Edition No
Laboratory Glassware n Edition No. 2 n Index Introduction 3 Ground joint glassware 13 Volumetric glassware 53 General laboratory glassware 65 Alphabetical index 76 Índice alfabético 77 Index Reference index 78 [email protected] Scharlau has been in the scientific glassware business for over 15 years Until now Scharlab S.L. had limited its sales to the Spanish market. However, now, coinciding with the inauguration of the new workshop next to our warehouse in Sentmenat, we are ready to export our scientific glassware to other countries. Standard and made to order Products for which there is regular demand are produced in larger Scharlau glassware quantities and then stocked for almost immediate supply. Other products are either manufactured directly from glass tubing or are constructed from a number of semi-finished products. Quality Even today, scientific glassblowing remains a highly skilled hand craft and the quality of glassware depends on the skill of each blower. Careful selection of the raw glass ensures that our final products are free from imperfections such as air lines, scratches and stones. You will be able to judge for yourself the workmanship of our glassware products. Safety All our glassware is annealed and made stress free to avoid breakage. Fax: +34 93 715 67 25 Scharlab The Lab Sourcing Group 3 www.scharlab.com Glassware Scharlau glassware is made from borosilicate glass that meets the specifications of the following standards: BS ISO 3585, DIN 12217 Type 3.3 Borosilicate glass ASTM E-438 Type 1 Class A Borosilicate glass US Pharmacopoeia Type 1 Borosilicate glass European Pharmacopoeia Type 1 Glass The typical chemical composition of our borosilicate glass is as follows: O Si 2 81% B2O3 13% Na2O 4% Al2O3 2% Glass is an inorganic substance that on cooling becomes rigid without crystallising and therefore it has no melting point as such. -

Atomic Layer Deposition on Dispersed Materials in Liquid Phase by Stoichiometrically Limited Injections
COMMUNICATION www.advmat.de Atomic Layer Deposition on Dispersed Materials in Liquid Phase by Stoichiometrically Limited Injections Benjamin P. Le Monnier, Frederick Wells, Farzaneh Talebkeikhah, and Jeremy S. Luterbacher* deposition.[2] Since then, processes for Atomic layer deposition (ALD) is a well-established vapor-phase technique depositing a range of materials including for depositing thin films with high conformality and atomically precise oxides, nitrides, hybrids and even metals [3–5] control over thickness. Its industrial development has been largely confined have been developed. This wide to wafers and low-surface-area materials because deposition on high-surface- variety of materials combined with its atomic-level precision has made ALD area materials and powders remains extremely challenging. Challenges with a formidable tool for the fabrication of such materials include long deposition times, extensive purging cycles, and nanostructured materials such as transis- requirements for large excesses of precursors and expensive low-pressure tors, solar cells and fuel cells.[6] equipment. Here, a simple solution-phase deposition process based on More recently, processes have been subsequent injections of stoichiometric quantities of precursor is performed developed to apply ALD to high-surface- area materials (>10 m2 g−1), including using common laboratory synthesis equipment. Precisely measured precursor powders for various applications, from stoichiometries avoid any unwanted reactions in solution and ensure layer-by- passivation of photoactive material to cata- layer growth with the same precision as gas-phase ALD, without any excess lyst preparation.[7,8] With such materials, precursor or purging required. Identical coating qualities are achieved when long exposure time (minutes vs millisec- onds for wafers), both for purge and reac- comparing this technique to Al2O3 deposition by fluidized-bed reactor ALD tion cycles, must be coupled with effec- (FBR-ALD). -
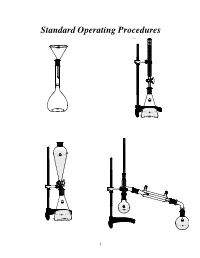
Standard Operating Procedures
Standard Operating Procedures 1 Standard Operating Procedures OVERVIEW In the following laboratory exercises you will be introduced to some of the glassware and tech- niques used by chemists to isolate components from natural or synthetic mixtures and to purify the individual compounds and characterize them by determining some of their physical proper- ties. While working collaboratively with your group members you will become acquainted with: a) Volumetric glassware b) Liquid-liquid extraction apparatus c) Distillation apparatus OBJECTIVES After finishing these sessions and reporting your results to your mentor, you should be able to: • Prepare solutions of exact concentrations • Separate liquid-liquid mixtures • Purify compounds by recrystallization • Separate mixtures by simple and fractional distillation 2 EXPERIMENT 1 Glassware Calibration, Primary and Secondary Standards, and Manual Titrations PART 1. Volumetric Glassware Calibration Volumetric glassware is used to either contain or deliver liquids at a specified temperature. Glassware manufacturers indicate this by inscribing on the volumetric ware the initials TC (to contain) or TD (to deliver) along with the calibration temperature, which is usually 20°C1. Volumetric glassware must be scrupulously clean before use. The presence of streaks or droplets is an indication of the presence of a grease film. To eliminate grease from glassware, scrub with detergent solution, rinse with tap water, and finally rinse with a small portion of distilled water. Volumetric flasks (TC) A volumetric flask has a large round bottom with only one graduation mark positioned on the long narrow neck. Graduation Mark Stopper The position of the mark facilitates the accurate and precise reading of the meniscus. If the flask is used to prepare a solution starting with a solid compound, add small amounts of sol- vent until the entire solid dissolves. -

A Simple Spectrophotometric Method for the Determination Of
J Clin Pathol: first published as 10.1136/jcp.14.2.202 on 1 March 1961. Downloaded from 202 Technical methods usual laboratory filters may give falsely increased values.) Of the clear and colourless filtrate 10 ml. is pipetted into A a 25 ml. measuring flask and 10 ml. reagent (3) added. simple spectrophotometric After mixing dilute with distilled water to the mark and method for the determination of renew mixing. After 10 minutes the mixture is ready for reading. The colour is stable, according to Watt and urea in blood and urine Chrisp, for 11 days. READING T. K. WITH, TOVE DREYER PETERSEN, AND BIRGIT PETERSEN From the Central Laboratory, Svend- The reactions are read in a spectrophotometer at 420 nm. borg County Hospital, Denmark with a blank prepared in the same way as the reaction mixture but with 3 ml. of distilled water instead of 3 ml. of blood. The urea standard (reagent 4) is treated in the Several methods for urea in determining blood and urine same way as the blood. Cuvettes of 1 cm. thickness are are in use in clinical laboratories, but none is ideal in suitable. mass routine The analysis. spectrophotometric method If p, s, and b are the extinctions of the the based on a modified Ehrlich sample, reagent proposed by Watt standard, and the blank respectively, the urea concen- and Chrisp (1954) for pure solutions is therefore of tration is interest because it can be modified for use in mass clinical analyses of blood and urine. Brown (1959) has l00(p-b)/(s-b) mg./100 ml. -

SC-300 Pro Kit Manual 1 Version 2.3D Materials Provided in the Kit
Vinmetrica SC-300 Pro Kit™ User Manual Vinmetrica SC-300 Pro Kit is a simple and robust device that provides high accuracy in determination of sulfite (SO2), pH and titratable acidity (TA) levels in wines, ciders, and other liquids. These are essential parameters to control in the effort to make high quality wines. The Pro kit includes lab accessories for the SC-300 Analyzer. Table of Contents: Materials Provided in the Kit............................................................................................................... 2 Things you will need............................................................................................................................ 2 Why Test for SO2, pH and TA?............................................................................................................ 3 Theory of Operation............................................................................................................................. 4 Setup.................................................................................................................................................... 5 Setting up the SC-300 for the first time................................................................................... 5 Assembling the Pro Kit Equipment......................................................................................... 6 Burette Maintenance................................................................................................................ 8 Burette Reading ...................................................................................................................... -

Environmental Chemistry Method Methoxyfenozide & Degradates In
Dow AgroSciences LLC Study ID: 110356 Page 12 Method Validation Study for the Detennination of Residues of Methoxyfenozide and its A-ring • Phenol Metabolite and B-ring Mono Acid Metabolite in Surface Water, Ground Water and Drinking Water by Liquid Chromatography with Tandem Mass Spectrometry INTRODUCTION J This method is applicable for the quantitative detennination of residues of methoxyfenozide and its A-ring phenol metabolite and B-ring mono acid metabolite in surface, ground and drinking water. The method was validated over the concentration range of 0.05-1.0 µg/L with a validated limit of quantitation of 0.05 µg/L. Common names, chemical names, and molecular formulas for the analytes are given in Table I. This study was conducted to fulfill data requirements outlined in the EPA Residue Chemistry Test Guidelines, OPPTS 850. 7100 (/). The validation will also comply with the requirements of EU Council Regulation (EC) No. 1107/2009 with particular regard to Section 3 of SANCO/3029/99 rev.4 and Section 3 of SANCO/825/00 rev.8.1 as well as PMRA Regulatory Directive Dir98-02 (2-4). The validation was conducted following Dow AgroSciences SOP ECL-24 with exceptions noted in the protocol or by protocol amendment. Method Principle Residues of methoxyfenozide, its A-ring phenol metabolite and its B-ring mono acid metabolite • are extracted from water samples by taking a l 0.0-mL aliquot and placing in an I I -dram (45-mL) glass vial equipped with a PTFE-lined cap or a 50-mL polypropylene centrifuge tube equipped with a cap. -

General Chemistry Laboratory I Manual
GENERAL CHEMISTRY LABORATORY I MANUAL Fall Semester Contents Laboratory Equipments .............................................................................................................................. i Experiment 1 Measurements and Density .............................................................................................. 10 Experiment 2 The Stoichiometry of a Reaction ..................................................................................... 31 Experiment 3 Titration of Acids and Bases ............................................................................................ 10 Experiment 4 Oxidation – Reduction Titration ..................................................................................... 49 Experiment 5 Quantitative Analysis Based on Gas Properties ............................................................ 57 Experiment 6 Thermochemistry: The Heat of Reaction ....................................................................... 67 Experiment 7 Group I: The Soluble Group ........................................................................................... 79 Experiment 8 Gravimetric Analysis ........................................................................................................ 84 Scores of the General Chemistry Laboratory I Experiments ............................................................... 93 LABORATORY EQUIPMENTS BEAKER (BEHER) Beakers are containers which can be used for carrying out reactions, heating solutions, and for water baths. They are for -

Laboratory Equipment Reference Sheet
Laboratory Equipment Stirring Rod: Reference Sheet: Iron Ring: Description: Glass rod. Uses: To stir combinations; To use in pouring liquids. Evaporating Dish: Description: Iron ring with a screw fastener; Several Sizes Uses: To fasten to the ring stand as a support for an apparatus Description: Porcelain dish. Buret Clamp/Test Tube Clamp: Uses: As a container for small amounts of liquids being evaporated. Glass Plate: Description: Metal clamp with a screw fastener, swivel and lock nut, adjusting screw, and a curved clamp. Uses: To hold an apparatus; May be fastened to a ring stand. Mortar and Pestle: Description: Thick glass. Uses: Many uses; Should not be heated Description: Heavy porcelain dish with a grinder. Watch Glass: Uses: To grind chemicals to a powder. Spatula: Description: Curved glass. Uses: May be used as a beaker cover; May be used in evaporating very small amounts of Description: Made of metal or porcelain. liquid. Uses: To transfer solid chemicals in weighing. Funnel: Triangular File: Description: Metal file with three cutting edges. Uses: To scratch glass or file. Rubber Connector: Description: Glass or plastic. Uses: To hold filter paper; May be used in pouring Description: Short length of tubing. Medicine Dropper: Uses: To connect parts of an apparatus. Pinch Clamp: Description: Glass tip with a rubber bulb. Uses: To transfer small amounts of liquid. Forceps: Description: Metal clamp with finger grips. Uses: To clamp a rubber connector. Test Tube Rack: Description: Metal Uses: To pick up or hold small objects. Beaker: Description: Rack; May be wood, metal, or plastic. Uses: To hold test tubes in an upright position. -

High School Chemistry
RECOMMENDED MINIMUM CORE INVENTORY TO SUPPORT STANDARDS-BASED INSTRUCTION HIGH SCHOOL GRADES SCIENCES High School Chemistry Quantity per Quantity per lab classroom/ Description group adjacent work area SAFETY EQUIPMENT 2 Acid storage cabinet (one reserved exclusively for nitric acid) 1 Chemical spill kit 1 Chemical storage reference book 5 Chemical waste containers (Categories: corrosives, flammables, oxidizers, air/water reactive, toxic) 1 Emergency shower 1 Eye wash station 1 Fire blanket 1 Fire extinguisher 1 First aid kit 1 Flammables cabinet 1 Fume hood 1/student Goggles 1 Goggles sanitizer (holds 36 pairs of goggles) 1/student Lab aprons COMPUTER ASSISTED LEARNING 1 Television or digital projector 1 VGA Adapters for various digital devices EQUIPMENT/SUPPLIES 1 box Aluminum foil 100 Assorted rubber stoppers 1 Balance, analytical (0.001g precision) 5 Balance, electronic or manual (0.01g precision) 1 pkg of 50 Balloons, latex 4 Beakers, 50 mL 4 Beakers, 100 mL 2 Beakers, 250 mL Developed by California Science Teachers Association to support the implementation of the California Next Generation Science Standards. Approved by the CSTA Board of Directors November 17, 2015. Quantity per Quantity per lab classroom/ Description group adjacent work area 2 Beakers, 400 or 600 mL 1 Beakers, 1000 mL 1 Beaker tongs 1 Bell jar 4 Bottle, carboy round, LDPE 10 L 4 Bottle, carboy round, LDPE 4 L 10 Bottle, narrow mouth, 1000 mL 20 Bottle, narrow mouth, 125 mL 20 Bottle, narrow mouth, 250 mL 20 Bottle, narrow mouth, 500 mL 10 Bottle, wide mouth, 125 -

Inquiry-Based Laboratory Work in Chemistry
INQUIRY-BASED LABORATORY WORK IN CHEMISTRY TEACHER’S GUIDE Derek Cheung Department of Curriculum and Instruction The Chinese University of Hong Kong Inquiry-based Laboratory Work in Chemistry: Teacher’s Guide / Derek Cheung Copyright © 2006 by Quality Education Fund, Hong Kong All rights reserved. Published by the Department of Curriculum and Instruction, The Chinese University of Hong Kong. No part of this book may be reproduced in any manner whatsoever without written permission, except in the case of use as instructional material in a school by a teacher. Note: The material in this teacher’s guide is for information only. No matter which inquiry-based lab activity teachers choose to try out, they should always conduct risks assessment in advance and highlight safety awareness before the lab begins. While every effort has been made in the preparation of this teacher’s guide to assure its accuracy, the Chinese University of Hong Kong and Quality Education Fund assume no liability resulting from errors or omissions in this teacher’s guide. In no event will the Chinese University of Hong Kong or Quality Education Fund be liable to users for any incidental, consequential or indirect damages resulting from the use of the information contained in the teacher’s guide. ISBN 962-85523-0-9 Printed and bound by Potential Technology and Internet Ltd. Contents Preface iv Teachers’ Concerns about Inquiry-based Laboratory Work 1 Secondary 4 – 5 Guided Inquiries 1. How much sodium bicarbonate is in one effervescent tablet? 4 2. What is the rate of a lightstick reaction? 18 3. Does toothpaste protect teeth? 29 4. -

Chm 204 F-14 Expt 13 Gases
Small-scale Preparation of CO2, H2, and O2. In this experiment you will learn a general method for generation of gases inside a large syringe. In addition to the three gases prepared today, the same method can be used to prepare a variety of other gases. Microscale Gas Chemistry Kits. Each pair of students will need certain equipment in order to prepare gases and perform experiments with the gases. We recommend organizing this equipment in 8-cup plastic food storage containers. Each kit should contain: v two 60 mL plastic syringes with a LuerLOK fitting v two Latex LuerLOK syringe caps v two plastic vial caps v one 15 cm length of Latex tubing v one 3 cm length of Latex tubing v one clear plastic beverage cup (250 mL/9 oz) v two small plastic weighing dishes v two small test tubes (12 x 100 mm) v glass pipet filled with copper wool v wooden splint v matches v wide-mouth beverage bottle (student supplied) v plastic pipet The In-Syringe Method for Preparing Gas Samples. The general strategy of the method is to react two substances in a 60 mL syringe. The limiting reagent is always used in solid form and is placed in a small vial cap. The second reagent is prepared as an aqueous solution. For example, one could generate CO2(g) from excess aqueous acetic acid and solid NaHCO3, as the limiting reagent. The solid reagent. The solid reagent is placed in the vial cap that is then lowered into the syringe barrel by water flotation. -

2017 Automated Liquid Handling Resource Guide
The Lab Manager AUTOMATED LIQUID HANDLING RESOURCE GUIDE What You Need to Know When Buying an Automated Liquid Handling System BY RYAN ACKERMAN Upgrading Stand-Alone Automated Liquid Handling Systems to Workstations BY ANGELO DEPALMA, PhD Small-Volume Liquid Handling Requires Advanced Technology BY MIKE MAY, PhD 20 10 Common Liquid Handling Errors & How to Avoid Them 17 BY LAB MANAGER The Latest in Pipette Tip Design BY ANGELO DEPALMA, PhD Automated Liquid Handling Product Finder BY LAB MANAGER Automatic Liquid Handling Resource Guide 2017 What You Need to Know When Buying an Automated Liquid Handling System Automated liquid handlers come in a seemingly endless variety of configurations, with many different specifications. By Ryan Ackerman MAINTENANCE TIP: AUTOMATED LIQUID HANDLING The signs that you should get your automated liquid handler serviced are fairly obvious. If the system is constantly experiencing glitches or producing inconsistent, unreliable results, it’s probably time to do some maintenance. Mechanical problems are another hint that you may want to call your service technician—these issues can include pipette tips being out of calibration, the deck not being “framed” correctly, belts being worn out, or the pipette stages being out of alignment. Why is it important to know the typical sample How does the sensitivity to contamination affect the type volume being used? of automated liquid handler required? Automated liquid handlers come in a seemingly endless variety of As the instrumentation used to analyze samples becomes more configurations, with many different specifications. An important one sensitive, the methods used to transport and prepare the samples to consider is what minimum, or maximum dispensing volume is must become resistant to contamination to ensure no carryover correct for your processes.