University of Florida Thesis Or Dissertation Formatting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Contrasting Patterns of Andean Diversification Among Three Diverse Clades of Neotropical Clearwing Butterflies
Contrasting patterns of Andean diversification among three diverse clades of Neotropical clearwing butterflies The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Chazot, Nicolas, Donna Lisa De#Silva, Keith R. Willmott, André V. L. Freitas, Gerardo Lamas, James Mallet, Carlos E. Giraldo, Sandra Uribe, and Marianne Elias. 2018. “Contrasting patterns of Andean diversification among three diverse clades of Neotropical clearwing butterflies.” Ecology and Evolution 8 (8): 3965-3982. doi:10.1002/ ece3.3622. http://dx.doi.org/10.1002/ece3.3622. Published Version doi:10.1002/ece3.3622 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:37160427 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Received: 12 April 2017 | Revised: 31 August 2017 | Accepted: 11 October 2017 DOI: 10.1002/ece3.3622 ORIGINAL RESEARCH Contrasting patterns of Andean diversification among three diverse clades of Neotropical clearwing butterflies Nicolas Chazot1,2,* | Donna Lisa De-Silva2,* | Keith R. Willmott3 | André V. L. Freitas4 | Gerardo Lamas5 | James Mallet6 | Carlos E. Giraldo7 | Sandra Uribe8 | Marianne Elias2 1Department of Biology, Lunds Universitet, Lund, Sweden 2Institut de Systématique, Évolution, Biodiversité, ISYEB-UMR 7205–CNRS MNHN UPMC EPHE, Muséum national -

Uehara-Prado Marcio D.Pdf
FICHA CATALOGRÁFICA ELABORADA PELA BIBLIOTECA DO INSTITUTO DE BIOLOGIA – UNICAMP Uehara-Prado, Marcio Ue3a Artrópodes terrestres como indicadores biológicos de perturbação antrópica / Marcio Uehara do Prado. – Campinas, SP: [s.n.], 2009. Orientador: André Victor Lucci Freitas. Tese (doutorado) – Universidade Estadual de Campinas, Instituto de Biologia. 1. Indicadores (Biologia). 2. Borboleta . 3. Artrópode epigéico. 4. Mata Atlântica. 5. Cerrados. I. Freitas, André Victor Lucci. II. Universidade Estadual de Campinas. Instituto de Biologia. III. Título. (rcdt/ib) Título em inglês: Terrestrial arthropods as biological indicators of anthropogenic disturbance. Palavras-chave em inglês : Indicators (Biology); Butterflies; Epigaeic arthropod; Mata Atlântica (Brazil); Cerrados. Área de concentração: Ecologia. Titulação: Doutor em Ecologia. Banca examinadora: André Victor Lucci Freitas, Fabio de Oliveira Roque, Paulo Roberto Guimarães Junior, Flavio Antonio Maës dos Santos, Thomas Michael Lewinsohn. Data da defesa : 21/08/2009. Programa de Pós-Graduação: Ecologia. iv Dedico este trabalho ao professor Keith S. Brown Jr. v AGRADECIMENTOS Ao longo dos vários anos da tese, muitas pessoas contribuiram direta ou indiretamente para a sua execução. Gostaria de agradecer nominalmente a todos, mas o espaço e a memória, ambos limitados, não permitem. Fica aqui o meu obrigado geral a todos que me ajudaram de alguma forma. Ao professor André V.L. Freitas, por sempre me incentivar e me apoiar em todos os momentos da tese, e por todo o ensinamento passado ao longo de nossa convivência de uma década. A minha família: Dona Júlia, Bagi e Bete, pelo apoio incondicional. A Cris, por ser essa companheira incrível, sempre cuidando muito bem de mim. A todas as meninas que participaram do projeto original “Artrópodes como indicadores biológicos de perturbação antrópica em Floresta Atlântica”, em especial a Juliana de Oliveira Fernandes, Huang Shi Fang, Mariana Juventina Magrini, Cristiane Matavelli, Tatiane Gisele Alves e Regiane Moreira de Oliveira. -

INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a Synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a Historical Sketch
ZOOLOGÍA-TAXONOMÍA www.unal.edu.co/icn/publicaciones/caldasia.htm Caldasia 31(2):407-440. 2009 HACIA UNA SÍNTESIS DE LOS PAPILIONOIDEA (INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a historical sketch JOSÉ LUIS SALINAS-GUTIÉRREZ El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] CLAUDIO MÉNDEZ Escuela de Biología, Universidad de San Carlos, Ciudad Universitaria, Campus Central USAC, Zona 12. Guatemala, Guatemala. [email protected] MERCEDES BARRIOS Centro de Estudios Conservacionistas (CECON), Universidad de San Carlos, Avenida La Reforma 0-53, Zona 10, Guatemala, Guatemala. [email protected] CARMEN POZO El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] JORGE LLORENTE-BOUSQUETS Museo de Zoología, Facultad de Ciencias, UNAM. Apartado Postal 70-399, México D.F. 04510; México. [email protected]. Autor responsable. RESUMEN La riqueza biológica de Mesoamérica es enorme. Dentro de esta gran área geográfi ca se encuentran algunos de los ecosistemas más diversos del planeta (selvas tropicales), así como varios de los principales centros de endemismo en el mundo (bosques nublados). Países como Guatemala, en esta gran área biogeográfi ca, tiene grandes zonas de bosque húmedo tropical y bosque mesófi lo, por esta razón es muy importante para analizar la diversidad en la región. Lamentablemente, la fauna de mariposas de Guatemala es poco conocida y por lo tanto, es necesario llevar a cabo un estudio y análisis de la composición y la diversidad de las mariposas (Lepidoptera: Papilionoidea) en Guatemala. -

Butterflies (Lepidoptera: Papilionoidea) in a Coastal Plain Area in the State of Paraná, Brazil
62 TROP. LEPID. RES., 26(2): 62-67, 2016 LEVISKI ET AL.: Butterflies in Paraná Butterflies (Lepidoptera: Papilionoidea) in a coastal plain area in the state of Paraná, Brazil Gabriela Lourenço Leviski¹*, Luziany Queiroz-Santos¹, Ricardo Russo Siewert¹, Lucy Mila Garcia Salik¹, Mirna Martins Casagrande¹ and Olaf Hermann Hendrik Mielke¹ ¹ Laboratório de Estudos de Lepidoptera Neotropical, Departamento de Zoologia, Universidade Federal do Paraná, Caixa Postal 19.020, 81.531-980, Curitiba, Paraná, Brazil Corresponding author: E-mail: [email protected]٭ Abstract: The coastal plain environments of southern Brazil are neglected and poorly represented in Conservation Units. In view of the importance of sampling these areas, the present study conducted the first butterfly inventory of a coastal area in the state of Paraná. Samples were taken in the Floresta Estadual do Palmito, from February 2014 through January 2015, using insect nets and traps for fruit-feeding butterfly species. A total of 200 species were recorded, in the families Hesperiidae (77), Nymphalidae (73), Riodinidae (20), Lycaenidae (19), Pieridae (7) and Papilionidae (4). Particularly notable records included the rare and vulnerable Pseudotinea hemis (Schaus, 1927), representing the lowest elevation record for this species, and Temenis huebneri korallion Fruhstorfer, 1912, a new record for Paraná. These results reinforce the need to direct sampling efforts to poorly inventoried areas, to increase knowledge of the distribution and occurrence patterns of butterflies in Brazil. Key words: Atlantic Forest, Biodiversity, conservation, inventory, species richness. INTRODUCTION the importance of inventories to knowledge of the fauna and its conservation, the present study inventoried the species of Faunal inventories are important for providing knowledge butterflies of the Floresta Estadual do Palmito. -

Aggregate?Pinheiro Et Al
610 Why do the ithomiines (Lepidoptera, Nymphalidae) aggregate?Pinheiro et al. Notes on a butterfly pocket in central Brazil 1 2 3 Carlos E. G. Pinheiro , Ísis Meri Medri & Ana Karina Moreyra Salcedo 1Departamento de Zoologia, Universidade de Brasília – UnB, 70910-900 Brasília-DF, Brasil. [email protected] 2,3Programa de Pós-graduação em Ecologia, Depto. de Ecologia, Universidade de Brasília – UnB, 70910-900 Brasília-DF, Brasil. [email protected] ABSTRACT. Why do the ithomiines (Lepidoptera, Nymphalidae) aggregate? Notes on a butterfly pocket in central Brazil. This study provides information on the species composition and the number of butterflies in different phases of an ithomiine aggregation during the 2004 dry season in central Brazil, and tests some hypotheses concerning the pocket formation. The results obtained suggest that ithomiine pockets constitute primarily an adaptation of butterflies to the adverse climatic conditions of the dry season, such as high temperatures and low air relative humidity, rather than the occurrence of large concentrations of adult food resources (flowers visited for nectar were not found in the pocket site) or defense against visually hunting predators (contrary to the prediction tested, the frequency of butterflies bearing birds beak marks on the wings significantly increased along the period of pocket formation, especially in the case of Mechanitis polymnia, the most abundant species in the pocket). Other hypotheses concerning the pocket formation are also discussed. KEYWORDS. Beak marks; insectivorous birds; ithomiine pockets; Müllerian mimicry. RESUMO. Por que os Ithomiinae (Lepidoptera, Nymphalidae) se agregam? Observações sobre um bolsão de borboletas no Brasil central. Este trabalho apresenta dados sobre a composição de espécies e o número de indivíduos encontrados em diferentes fases de formação de um bolsão de Ithomiinae investigado na estação seca de 2004 em uma floresta de galeria do Brasil central, e testa algumas hipóteses relacionadas à formação do bolsão. -
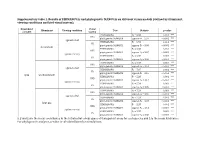
Supplementary Table 1. Results of Permanovas and Phylogenetic Manovas on Different Vision Models (Defined by Illuminant, Viewing Conditions and Bird Visual System)
Supplementary table 1. Results of PERMANOVAs and phylogenetic MANOVAs on different vision models (defined by illuminant, viewing conditions and bird visual system). Dependent Visual Illuminant Viewing condition Test Statistic p-value variable system PERMANOVA F9 = 6.88 0.001 *** UVS phylogenetic MANOVA approx-F9 = 2.97 < 0.001 *** against a leaf PERMANOVA F9 = 6.93 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.05 < 0.001 *** forest shade PERMANOVA F9 = 5.38 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** against the sky PERMANOVA F9 = 5.38 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.36 < 0.001 *** PERMANOVA F9 = 7.04 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.01 < 0.001 *** against a leaf PERMANOVA F9 = 7.07 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.10 < 0.001 *** xyzL woodland shade PERMANOVA F9 = 5.33 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.12 < 0.001 *** against the sky PERMANOVA F9 = 5.34 0.002 ** VS phylogenetic MANOVA approx-F9 = 3.39 < 0.001 *** PERMANOVA F9 = 7.24 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.00 < 0.001 *** against a leaf PERMANOVA F9 = 7.24 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** large gap PERMANOVA F9 = 5.37 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.14 < 0.001 *** against the sky PERMANOVA F9 = 5.37 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.38 < 0.001 *** x, y and z are the mean coordinates in the tetrahedral colour space of transparent areas for each species and L is the mean luminance. -

Rev Iss Web Mec 13773 25-22 5765..5784
Molecular Ecology (2016) 25, 5765–5784 doi: 10.1111/mec.13773 Into the Andes: multiple independent colonizations drive montane diversity in the Neotropical clearwing butterflies Godyridina NICOLAS CHAZOT,*† KEITH R. WILLMOTT,‡ FABIEN L. CONDAMINE,§¶ DONNA LISA DE-SILVA,* ANDREV.L.FREITAS,**GERARDOLAMAS,†† HELENE MORLON,‡‡ CARLOS E. GIRALDO,§§ CHRIS D. JIGGINS,¶¶ MATHIEU JORON,*** JAMES MALLET,††† SANDRA URIBE‡‡‡ and MARIANNE ELIAS* *Institut de Systematique, Evolution, Biodiversite, ISYEB – UMR 7205 – CNRS MNHN UPMC EPHE, Museum national d’Histoire naturelle, Sorbonne Universites, 57 rue Cuvier CP50, F-75005 Paris, France, †Department of Biology, University of Lund, 223 62 Lund, Sweden, ‡McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, Gainesville, FL 32611, USA, §CNRS, UMR 5554 Institut des Sciences de l’Evolution (Universite de Montpellier), Place Eugene Bataillon, 34095 Montpellier, France, ¶Department of Biological Sciences, University of Alberta, T6G 2E9 Edmonton, AB, Canada, **Departamento de Zoologia and Museu de Zoologia, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, S~ao Paulo, Brazil, ††Museo de Historia Natural, Universidad Nacional de San Marcos, Lima, Peru, ‡‡IBENS, Ecole Normale Superieure, UMR 8197 CNRS, Paris, France, §§Grupo de Investigacion de Sanidad Vegetal, Universidad Catolica de Oriente, Rionegro, Antioquia, Colombia, ¶¶Department of Zoology, University of Cambridge, Cambridge, UK, ***Centre d’Ecologie Fonctionnelle et Evolutive, CEFE, UMR 5175 CNRS – EPHE – Universite de Montpellier – Universite Paul Valery Montpellier, 34293 Montpellier 5, France, †††Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138, USA, ‡‡‡Universidad Nacional de Colombia, sede Medellın, Medellın, Colombia Abstract Understanding why species richness peaks along the Andes is a fundamental question in the study of Neotropical biodiversity. -

Molecular Phylogenetics of the Neotropical Butterfly Subtribe Oleriina
Molecular Phylogenetics and Evolution 55 (2010) 1032–1041 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Molecular phylogenetics of the neotropical butterfly subtribe Oleriina (Nymphalidae: Danainae: Ithomiini) Donna Lisa de-Silva a,*, Julia J. Day a, Marianne Elias b,c, Keith Willmott d, Alaine Whinnett a, James Mallet a a Department of Genetics, Evolution and Environment, University College London, Wolfson House, 4 Stephenson Way, London NW1 2HE, UK b Imperial College London, Silwood Park, Buckhurst Road, Ascot, Berkshire SL5 7PY, UK c CNRS, UMR 7205, Muséum National d’Histoire Naturelle, 45 Rue Buffon, CP50, 75005 Paris, France d McGuire Center for Lepidoptera, Florida Museum of Natural History, University of Florida, P.O. Box 112710, Gainesville, FL 32611-2710, USA article info abstract Article history: The Oleriina is one of the most speciose subtribes of the neotropical nymphalid butterfly tribe Ithomiini. Received 9 September 2009 They are widely distributed across the Andes and Amazonian lowlands and like other ithomiines they are Revised 22 December 2009 involved in complex mimicry rings. This subtribe is of particular interest because it contains the most Accepted 9 January 2010 diverse ithomiine genus, Oleria, as well as two genera, Megoleria and Hyposcada, that feed on hostplants Available online 15 January 2010 not utilized elsewhere in the tribe. Here we present the first comprehensive species-level phylogeny for the Oleriina, representing 83% of recognised species in the group, and based on 6698 bp from eight mito- Keywords: chondrial (mt) and nuclear (nc) genes. Topologies are largely congruent for ncDNA and the concatenated Lepidoptera dataset and the genera Oleria, Hyposcada and Megoleria are recovered and well-supported, although Speciation Phylogeny strongly discordant genealogy between mtDNA and ncDNA suggest possible introgression among Hypos- Hybridization cada and Megoleria. -

Borboletas (Lepidoptera) Ameaçadas De Extinção Em Minas Gerais, Brasil 1
BORBOLETAS (LEPIDOPTERA) AMEAÇADAS DE EXTINÇÃO EM MINAS GERAIS, BRASIL 1 Mirna M. Casagrande 2 Olaf H.H. Mielke 2 Keith S. Brown Jr. 3 ABSTRACT. BUTTERFLlES (LEPIDOPTERA) CONS IDERED AS THR EATENED lN MINAS GERAI S, BRA ZIL. The twenty species ofbutterflies (diurnal Lepidoptera) considered as threatened in the Minas Gerais (by statute) are described and di scussed in relation to di stribution, appearance and known records. KEY WORDS. Lepidoptera, butterflies threatened, Brazil o presente trabalho visa ilustrar as espécies incluídas na "Lista de espécies ameaçadas de extinção do Estado de Minas Gerais" publicada pela COPAM (Conselho Estadual de Política Ambiental) (MINAS GERAIS 1996). Detalhes com informações ecológicas serão incluídos no "Livro vermelho das espécies ameaçadas do estado de Minas Gerais" a ser editado pela Fundação Biodiversitas. As borboletas pertencem à ordem Lepidoptera que compreende aproxima damente 150.000 espécies conhecidas, das quais 19.000 são borboletas (HEPPNER 1991), sendo que no Brasil devem ocorrer ao todo 40.000 espécies, das quais 3.300 espécies de borboletas (BROWN I 996a,b). As borboletas são quase todas diurnas, com algumas poucas exceções (Hesperiidae, Lycaenidae e Nymphalidae: Satyrinae) e se diferenciam das mariposas pelas antenas clavadas e nunca terem um frênulo no ângulo umeral da asa posterior acoplado ao retináculo na face ventral da asa anterior (uma só exceção na Austrália com frênulo - Euschemon rajjl.esia Macleay, 1827, Hesperiidae). As borboletas são mais bem conhecidas que as mariposas e é possível reconhecer algumas espécies como consideradas ameaçadas de extinção, na maioria dos casos por destruição do seu habitat típico pelo avanço dos sistemas antrópicos que já substituiram mais de 90% dos sistemas naturais e 95% da Floresta Atlântica no estado de Minas Gerais. -

Effects of Land Use on Butterfly (Lepidoptera: Nymphalidae) Abundance and Diversity in the Tropical Coastal Regions of Guyana and Australia
ResearchOnline@JCU This file is part of the following work: Sambhu, Hemchandranauth (2018) Effects of land use on butterfly (Lepidoptera: Nymphalidae) abundance and diversity in the tropical coastal regions of Guyana and Australia. PhD Thesis, James Cook University. Access to this file is available from: https://doi.org/10.25903/5bd8e93df512e Copyright © 2018 Hemchandranauth Sambhu The author has certified to JCU that they have made a reasonable effort to gain permission and acknowledge the owners of any third party copyright material included in this document. If you believe that this is not the case, please email [email protected] EFFECTS OF LAND USE ON BUTTERFLY (LEPIDOPTERA: NYMPHALIDAE) ABUNDANCE AND DIVERSITY IN THE TROPICAL COASTAL REGIONS OF GUYANA AND AUSTRALIA _____________________________________________ By: Hemchandranauth Sambhu B.Sc. (Biology), University of Guyana, Guyana M.Sc. (Res: Plant and Environmental Sciences), University of Warwick, United Kingdom A thesis Prepared for the College of Science and Engineering, in partial fulfillment of the requirements for the degree of Doctor of Philosophy James Cook University February, 2018 DEDICATION ________________________________________________________ I dedicate this thesis to my wife, Alliea, and to our little girl who is yet to make her first appearance in this world. i ACKNOWLEDGEMENTS ________________________________________________________ I would like to thank the Australian Government through their Department of Foreign Affairs and Trade for graciously offering me a scholarship (Australia Aid Award – AusAid) to study in Australia. From the time of my departure from my home country in 2014, Alex Salvador, Katherine Elliott and other members of the AusAid team have always ensured that the highest quality of care was extended to me as a foreign student in a distant land. -

1 ACTUALIDADES BIOLOGICAS V33 N95.Indd
Muriel et al. Actual Biol 33 (95): 275-285, 2011 NUEVOS REGISTROS DE PLANTAS HOSPEDERAS Y DISPONIBILIDAD DE RECURSOS PARA MARIPOSAS ITHOMIINI (LEPIDOPTERA: NYMPHALIDAE: DANAINAE), EN AGROECOSISTEMAS DE CAFÉ COLOMBIANOS NEW HOST PLANT RECORDS AND RESOURCE AVAILABILITY TO ITHOMIINI BUTTERFLIES (LEPIDOPTERA: NYMPHALIDAE: DANAINAE), IN COLOMBIAN COFFEE AGROECOSYSTEMS Sandra B. Muriel1, 3, Jorge Montoya2, 4, Alejandra Restrepo1, 5, Jonathan Muñoz1, 6 Resumen En el trópico se dispone de pocos datos de la mayoría de los organismos, incluyendo las mariposas, en aspectos claves de su ciclo de vida, sus plantas hospederas y factores explicativos de su diversidad y abundancia. El objetivo de este trabajo fue identificar las plantas hospederas de larvas Ithomiini (Lepidoptera) en agroecosistemas de café y evaluar el efecto de las variables: sistema de producción, área en bosque y diversidad de hospederas sobre la diversidad y abundancia de este grupo. En seis fincas de café de Fredonia (Antioquia), Colombia, se recolectaron plantas de las familias Apocynaceae, Gesneriaceae y Solanaceae, que fueron identificadas en los Herbarios HUA y MEDEL de Medellín. En las fincas se registraron los adultos observados en vuelo y se recolectaron huevos, larvas y pupas de Ithomiini de sus plantas hospederas para su cría en laboratorio, hasta la emergencia de adultos. Se determinó el porcentaje de sobrevivencia y mortalidad debida a parasitoidismo. Los datos de diversidad se analizaron por medio de un Análisis de Regresión de Poisson. En los agroecosistemas de café se registraron 27 especies vegetales y 27 mariposas adultas Ithomiini, en laboratorio, se criaron 326 individuos de ocho especies, recolectados sobre siete plantas de la familia Solanaceae. -

Nymphalidae: Ithomiinae)
STUDIES ON THE ECOLOGY AND EVOLUTION OF NEOTROPICAL ITHOMIINE BUTTERFLIES (NYMPHALIDAE: ITHOMIINAE) by GEORGE WILLIAM BECCALONI A thesis submitted for the degree of Doctor ofPhilosophy ofthe University ofLondon October 1995 Biogeography and Conservation Laboratory Centre for Population Biology Department of Entomology Imperial College The Natural History Museum Silwood Park Cromwell Road Ascot London SW7 5BD Berkshire SL5 7PY 2 To my mother, Benjie & Judy in love and gratitude 3 ABSTRACT Two aspects ofthe ecology ofNeotropical ithomiine butterflies (Nymphalidae: Ithomiinae) are discussed: mimicry (Chapters 2, 3) and species richness (Chapters 4, 5). Chapter 2 defines eight mimicry complexes involving ithomiines and other insects found in eastern Ecuador. These complexes are dominated by ithomiine individuals. Hypotheses to explain polymorphism in Batesian and Mullerian mimics are assessed. In Chapter 3, evidence that sympatric ithomiine-dominated mimicry complexes are segregated by microhabitat is reviewed. Data confirm that sympatric complexes are segregated vertically by flight height. Flight height is shown to be positively correlated with larval host-plant height. Host-plant partitioning between species in a butterfly community results in the formation of microhabitat guilds of species, and evidence suggests that mimicry may evolve between species which share a guild, but not between guilds. Models for the evolution of mimicry complexes in sympatry, and for polymorphism and dual sex-limited mimicry in Mullerian mimics, are discussed in the light of these findings. Chapter 4 investigates relationships between species richness offamilies and subfamilies ofNeotropical butterflies and overall butterfly species richness at local and regional scales. A strong positive correlation is demonstrated between ithomiine richness and the species richness of all other butterflies.