Molecular Phylogenetics of the Neotropical Butterfly Subtribe Oleriina
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alfred Russel Wallace and the Darwinian Species Concept
Gayana 73(2): Suplemento, 2009 ISSN 0717-652X ALFRED RUSSEL WALLACE AND THE Darwinian SPECIES CONCEPT: HIS paper ON THE swallowtail BUTTERFLIES (PAPILIONIDAE) OF 1865 ALFRED RUSSEL WALLACE Y EL concepto darwiniano DE ESPECIE: SU TRABAJO DE 1865 SOBRE MARIPOSAS papilio (PAPILIONIDAE) Jam ES MA LLET 1 Galton Laboratory, Department of Biology, University College London, 4 Stephenson Way, London UK, NW1 2HE E-mail: [email protected] Abstract Soon after his return from the Malay Archipelago, Alfred Russel Wallace published one of his most significant papers. The paper used butterflies of the family Papilionidae as a model system for testing evolutionary hypotheses, and included a revision of the Papilionidae of the region, as well as the description of some 20 new species. Wallace argued that the Papilionidae were the most advanced butterflies, against some of his colleagues such as Bates and Trimen who had claimed that the Nymphalidae were more advanced because of their possession of vestigial forelegs. In a very important section, Wallace laid out what is perhaps the clearest Darwinist definition of the differences between species, geographic subspecies, and local ‘varieties.’ He also discussed the relationship of these taxonomic categories to what is now termed ‘reproductive isolation.’ While accepting reproductive isolation as a cause of species, he rejected it as a definition. Instead, species were recognized as forms that overlap spatially and lack intermediates. However, this morphological distinctness argument breaks down for discrete polymorphisms, and Wallace clearly emphasised the conspecificity of non-mimetic males and female Batesian mimetic morphs in Papilio polytes, and also in P. -

North Andean Origin and Diversification of the Largest Ithomiine Butterfly Genus
North Andean origin and diversification of the largest ithomiine butterfly genus The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Lisa De-Silva, D., L. L. Mota, N. Chazot, R. Mallarino, K. L. Silva- Brandão, L. M. G. Piñerez, A. V. Freitas, et al. 2017. “North Andean origin and diversification of the largest ithomiine butterfly genus.” Scientific Reports 7 (1): 45966. doi:10.1038/srep45966. http:// dx.doi.org/10.1038/srep45966. Published Version doi:10.1038/srep45966 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:32630680 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA www.nature.com/scientificreports OPEN North Andean origin and diversification of the largest ithomiine butterfly genus Received: 31 October 2016 Donna Lisa De-Silva1, Luísa L. Mota2, Nicolas Chazot1,3, Ricardo Mallarino4, Karina L. Silva- Accepted: 22 February 2017 Brandão5, Luz Miryam Gómez Piñerez6,7, André V.L. Freitas2, Gerardo Lamas8, Published: 07 April 2017 Mathieu Joron9, James Mallet4, Carlos E. Giraldo6,10, Sandra Uribe6, Tiina Särkinen11, Sandra Knapp12, Chris D. Jiggins13, Keith R. Willmott14 & Marianne Elias1 The Neotropics harbour the most diverse flora and fauna on Earth. The Andes are a major centre of diversification and source of diversity for adjacent areas in plants and vertebrates, but studies on insects remain scarce, even though they constitute the largest fraction of terrestrial biodiversity. -

Developmental, Cellular and Biochemical Basis of Transparency in Clearwing Butterflies Aaron F
© 2021. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2021) 224, jeb237917. doi:10.1242/jeb.237917 RESEARCH ARTICLE Developmental, cellular and biochemical basis of transparency in clearwing butterflies Aaron F. Pomerantz1,2,*, Radwanul H. Siddique3,4, Elizabeth I. Cash5, Yuriko Kishi6,7, Charline Pinna8, Kasia Hammar2, Doris Gomez9, Marianne Elias8 and Nipam H. Patel1,2,6,* ABSTRACT INTRODUCTION The wings of butterflies and moths (Lepidoptera) are typically covered The wings of butterflies and moths (Lepidoptera) have inspired with thousands of flat, overlapping scales that endow the wings with studies across a variety of scientific fields, including evolutionary colorful patterns. Yet, numerous species of Lepidoptera have evolved biology, ecology and biophysics (Beldade and Brakefield, 2002; highly transparent wings, which often possess scales of altered Prum et al., 2006; Gilbert and Singer, 1975). Lepidopteran wings morphology and reduced size, and the presence of membrane are generally covered with rows of flat, partially overlapping surface nanostructures that dramatically reduce reflection. Optical scales that endow the wings with colorful patterns. Adult scales are properties and anti-reflective nanostructures have been characterized chitin-covered projections that serve as the unit of color for the wing. for several ‘clearwing’ Lepidoptera, but the developmental processes Each scale can generate color through pigmentation via molecules underlying wing transparency are unknown. Here, we applied that selectively absorb certain wavelengths of light, structural confocal and electron microscopy to create a developmental time coloration, which results from light interacting with the physical series in the glasswing butterfly, Greta oto, comparing transparent nanoarchitecture of the scale; or a combination of both pigmentary and non-transparent wing regions. -

A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1967 A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Ross, Gary Noel, "A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico." (1967). LSU Historical Dissertations and Theses. 1315. https://digitalcommons.lsu.edu/gradschool_disstheses/1315 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 67-14,010 ROSS, Gary Noel, 1940- A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO. Louisiana State University and Agricultural and Mechanical CoUege, Ph.D., 1967 Entomology University Microfilms, Inc., Ann Arbor, Michigan A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO A D issertation Submitted to the Graduate Faculty of the Louisiana State University and A gricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Entomology by Gary Noel Ross M.S., Louisiana State University, 196*+ May, 1967 FRONTISPIECE Section of the south wall of the crater of Volcan Santa Marta. May 1965, 5,100 feet. ACKNOWLEDGMENTS Many persons have contributed to and assisted me in the prep aration of this dissertation and I wish to express my sincerest ap preciation to them all. -

INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a Synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a Historical Sketch
ZOOLOGÍA-TAXONOMÍA www.unal.edu.co/icn/publicaciones/caldasia.htm Caldasia 31(2):407-440. 2009 HACIA UNA SÍNTESIS DE LOS PAPILIONOIDEA (INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a historical sketch JOSÉ LUIS SALINAS-GUTIÉRREZ El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] CLAUDIO MÉNDEZ Escuela de Biología, Universidad de San Carlos, Ciudad Universitaria, Campus Central USAC, Zona 12. Guatemala, Guatemala. [email protected] MERCEDES BARRIOS Centro de Estudios Conservacionistas (CECON), Universidad de San Carlos, Avenida La Reforma 0-53, Zona 10, Guatemala, Guatemala. [email protected] CARMEN POZO El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] JORGE LLORENTE-BOUSQUETS Museo de Zoología, Facultad de Ciencias, UNAM. Apartado Postal 70-399, México D.F. 04510; México. [email protected]. Autor responsable. RESUMEN La riqueza biológica de Mesoamérica es enorme. Dentro de esta gran área geográfi ca se encuentran algunos de los ecosistemas más diversos del planeta (selvas tropicales), así como varios de los principales centros de endemismo en el mundo (bosques nublados). Países como Guatemala, en esta gran área biogeográfi ca, tiene grandes zonas de bosque húmedo tropical y bosque mesófi lo, por esta razón es muy importante para analizar la diversidad en la región. Lamentablemente, la fauna de mariposas de Guatemala es poco conocida y por lo tanto, es necesario llevar a cabo un estudio y análisis de la composición y la diversidad de las mariposas (Lepidoptera: Papilionoidea) en Guatemala. -

Butterflies (Lepidoptera: Papilionoidea) in a Coastal Plain Area in the State of Paraná, Brazil
62 TROP. LEPID. RES., 26(2): 62-67, 2016 LEVISKI ET AL.: Butterflies in Paraná Butterflies (Lepidoptera: Papilionoidea) in a coastal plain area in the state of Paraná, Brazil Gabriela Lourenço Leviski¹*, Luziany Queiroz-Santos¹, Ricardo Russo Siewert¹, Lucy Mila Garcia Salik¹, Mirna Martins Casagrande¹ and Olaf Hermann Hendrik Mielke¹ ¹ Laboratório de Estudos de Lepidoptera Neotropical, Departamento de Zoologia, Universidade Federal do Paraná, Caixa Postal 19.020, 81.531-980, Curitiba, Paraná, Brazil Corresponding author: E-mail: [email protected]٭ Abstract: The coastal plain environments of southern Brazil are neglected and poorly represented in Conservation Units. In view of the importance of sampling these areas, the present study conducted the first butterfly inventory of a coastal area in the state of Paraná. Samples were taken in the Floresta Estadual do Palmito, from February 2014 through January 2015, using insect nets and traps for fruit-feeding butterfly species. A total of 200 species were recorded, in the families Hesperiidae (77), Nymphalidae (73), Riodinidae (20), Lycaenidae (19), Pieridae (7) and Papilionidae (4). Particularly notable records included the rare and vulnerable Pseudotinea hemis (Schaus, 1927), representing the lowest elevation record for this species, and Temenis huebneri korallion Fruhstorfer, 1912, a new record for Paraná. These results reinforce the need to direct sampling efforts to poorly inventoried areas, to increase knowledge of the distribution and occurrence patterns of butterflies in Brazil. Key words: Atlantic Forest, Biodiversity, conservation, inventory, species richness. INTRODUCTION the importance of inventories to knowledge of the fauna and its conservation, the present study inventoried the species of Faunal inventories are important for providing knowledge butterflies of the Floresta Estadual do Palmito. -

Archiv Für Naturgeschichte
© Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at Lepidoptera für 1903. Bearbeitet von Dr. Robert Lucas in Rixdorf bei Berlin. A. Publikationen (Autoren alphabetisch) mit Referaten. Adkin, Robert. Pyrameis cardui, Plusia gamma and Nemophila noc- tuella. The Entomologist, vol. 36. p. 274—276. Agassiz, G. Etüde sur la coloration des ailes des papillons. Lausanne, H. Vallotton u. Toso. 8 °. 31 p. von Aigner-Abafi, A. (1). Variabilität zweier Lepidopterenarten. Verhandlgn. zool.-bot. Ges. Wien, 53. Bd. p. 162—165. I. Argynnis Paphia L. ; IL Larentia bilineata L. — (2). Protoparce convolvuli. Entom. Zeitschr. Guben. 17. Jahrg. p. 22. — (3). Über Mimikry. Gaea. 39. Jhg. p. 166—170, 233—237. — (4). A mimicryröl. Rov. Lapok, vol. X, p. 28—34, 45—53 — (5). A Mimicry. Allat. Kozl. 1902, p. 117—126. — (6). (Über Mimikry). Allgem. Zeitschr. f. Entom. 7. Bd. (Schluß p. 405—409). Über Falterarten, welche auch gesondert von ihrer Umgebung, in ruhendem Zustande eine eigentümliche, das Auge täuschende Form annehmen (Lasiocampa quercifolia [dürres Blatt], Phalera bucephala [zerbrochenes Ästchen], Calocampa exoleta [Stück morschen Holzes]. — [Stabheuschrecke, Acanthoderus]. Raupen, die Meister der Mimikry sind. Nachahmung anderer Tiere. Die Mimik ist in vielen Fällen zwecklos. — Die wenn auch recht geistreichen Mimikry-Theorien sind doch vielleicht nur ein müßiges Spiel der Phantasie. Aitken u. Comber, E. A list of the butterflies of the Konkau. Journ. Bombay Soc. vol. XV. p. 42—55, Suppl. p. 356. Albisson, J. Notes biologiques pour servir ä l'histoire naturelle du Charaxes jasius. Bull. Soc. Etud. Sc. nat. Nimes. T. 30. p. 77—82. Annandale u. Robinson. Siehe unter S w i n h o e. -

Front Matter
Cambridge University Press 978-0-521-88318-4 - Speciation and Patterns of Diversity Edited by Roger K. Butlin, Jon R. Bridle and Dolph Schluter Frontmatter More information Speciation and Patterns of Diversity Bringing together the viewpoints of leading ecologists concerned with the processes that generate patterns of diversity, and evolutionary biologists who focus on mechanisms of speciation, this book opens up discussion in order to broaden understanding of how speciation affects patterns of biological diversity, especially the uneven distribution of diversity across time, space and taxa studied by macroecologists. The contributors discuss questions such as: Are species equivalent units, providing meaningful measures of diversity? To what extent do mechanisms of speciation affect the functional nature and distribution of species diversity? How can speciation rates be measured using molecular phylogenies or data from the fossil record? What are the factors that explain variation in rates? Written for graduate students and academic researchers, the book promotes a more complete understanding of the interaction between mechanisms and rates of speciation and these patterns in biological diversity. R OGER B UTLIN is Professor of Evolutionary Biology, Animal and Plant Sciences, at the University of Sheffield. He has held a prestigious Royal Society Research Fellowship at the University of Cardiff and his work has been recognized by honorary fellowships at the Natural History Museum, Zoological Society of London, and Royal Belgian Institute of Natural Sciences. J ON B RIDLE is Lecturer in Biology, in the School of Biological Sciences, University of Bristol. Since completing his PhD in Evolutionary Genetics in 1998, he has conducted research in quantitative genetics and evolutionary biology at University College London, University of Cardiff, Universidad Auto´ noma de Madrid, and the Institute of Zoology, London. -

Developmental, Cellular, and Biochemical
Developmental, cellular, and biochemical basis of transparency in the glasswing butterfly Greta oto Aaron Pomerantz, Radwanul Siddique, Elizabeth Cash, Yuriko Kishi, Charline Pinna, Kasia Hammar, Doris Gomez, Marianne Elias, Nipam Patel To cite this version: Aaron Pomerantz, Radwanul Siddique, Elizabeth Cash, Yuriko Kishi, Charline Pinna, et al.. Devel- opmental, cellular, and biochemical basis of transparency in the glasswing butterfly Greta oto. 2020. hal-03012452 HAL Id: hal-03012452 https://hal.archives-ouvertes.fr/hal-03012452 Preprint submitted on 18 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. bioRxiv preprint doi: https://doi.org/10.1101/2020.07.02.183590; this version posted July 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Title 2 Developmental, cellular, and biochemical basis of transparency in the glasswing butterfly 3 Greta oto 4 5 Authors 6 Aaron F. Pomerantz1,2*, Radwanul H. Siddique3,4, Elizabeth I. Cash5, Yuriko Kishi6,7, 7 Charline Pinna8, Kasia Hammar2, Doris Gomez9, Marianne Elias8, Nipam H. -
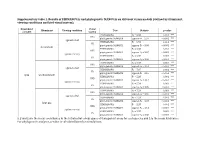
Supplementary Table 1. Results of Permanovas and Phylogenetic Manovas on Different Vision Models (Defined by Illuminant, Viewing Conditions and Bird Visual System)
Supplementary table 1. Results of PERMANOVAs and phylogenetic MANOVAs on different vision models (defined by illuminant, viewing conditions and bird visual system). Dependent Visual Illuminant Viewing condition Test Statistic p-value variable system PERMANOVA F9 = 6.88 0.001 *** UVS phylogenetic MANOVA approx-F9 = 2.97 < 0.001 *** against a leaf PERMANOVA F9 = 6.93 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.05 < 0.001 *** forest shade PERMANOVA F9 = 5.38 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** against the sky PERMANOVA F9 = 5.38 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.36 < 0.001 *** PERMANOVA F9 = 7.04 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.01 < 0.001 *** against a leaf PERMANOVA F9 = 7.07 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.10 < 0.001 *** xyzL woodland shade PERMANOVA F9 = 5.33 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.12 < 0.001 *** against the sky PERMANOVA F9 = 5.34 0.002 ** VS phylogenetic MANOVA approx-F9 = 3.39 < 0.001 *** PERMANOVA F9 = 7.24 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.00 < 0.001 *** against a leaf PERMANOVA F9 = 7.24 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** large gap PERMANOVA F9 = 5.37 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.14 < 0.001 *** against the sky PERMANOVA F9 = 5.37 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.38 < 0.001 *** x, y and z are the mean coordinates in the tetrahedral colour space of transparent areas for each species and L is the mean luminance. -

Elevation in Tropical Sky Islands As the Common Driver in Structuring Genes and Communities of Freshwater Organisms
Research Collection Journal Article Elevation in tropical sky islands as the common driver in structuring genes and communities of freshwater organisms Author(s): Gueuning, Morgan; Suchan, Tomasz; Rutschmann, Sereina; Gattolliat, Jean-Luc; Jamsari, Jamsari; Kamil, Al I.; Pitteloud, Camille; Buerki, Sven; Balke, Michael; Sartori, Michel; Alvarez, Nadir Publication Date: 2017 Permanent Link: https://doi.org/10.3929/ethz-b-000217957 Originally published in: Scientific Reports 7(1), http://doi.org/10.1038/s41598-017-16069-y Rights / License: Creative Commons Attribution 4.0 International This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library www.nature.com/scientificreports OPEN Elevation in tropical sky islands as the common driver in structuring genes and communities of Received: 30 January 2017 Accepted: 7 November 2017 freshwater organisms Published: xx xx xxxx Morgan Gueuning1,2, Tomasz Suchan1,12, Sereina Rutschmann1,3, Jean-Luc Gattolliat1,4, Jamsari Jamsari5, Al Ihsan Kamil5, Camille Pitteloud1,6,7, Sven Buerki8,9, Michael Balke10, Michel Sartori 1,4 & Nadir Alvarez 1,11 Tropical mountains are usually characterized by a vertically-arranged sequence of ecological belts, which, in contrast to temperate habitats, have remained relatively stable in space across the Quaternary. Such long-lasting patterning of habitats makes them ideal to test the role of environmental pressure in driving ecological and evolutionary processes. Using Sumatran freshwater mayfy communities, we test whether elevation, rather than other spatial factors (i.e. volcanoes, watersheds) structures both species within communities and genes within species. Based on the analysis of 31 mayfy (Ephemeroptera) communities and restriction-site-associated-DNA sequencing in the four most ubiquitous species, we found elevation as the major spatial component structuring both species and genes in the landscape. -

Panama's Brilliant Butterflies December 3-15, 2017
Panama's Brilliant Butterflies December 3-15, 2017 Guides: Faustino "Tino" Sanchez Daily Itinerary : Linda Harrison Day 1 - December 3rd; Arrival, Canopy Lodge gardens Day 2 - December 4th; Cara Iguana am & Las Mozas pm Day 3 - December 5th; Las Minas/Cara Iguana am & Finca Macarena/Las Minas pm TOTAL SPECIES: 301 (including 5?) Day 4 - December 6th; Altos del Maria & Canopy Lodge gardens Day 5 - December 7th; Canopy Lodge am & Canopy Tower/Semaphore Hill pm Main Tour: 235 Day 6 - December 8th; Ammo Ponds & Pipeline Road Day 7 - December 9th; Metropolitan Park, Canopy Tower & Summit Ponds Camp: 144 Day 8 - December 10th; Canopy Tower Canopy Camp Extension : Blue=species added Main Tour: 6 Day 8 - December 10th; Bayano Lake, Torti & Canopy Camp grounds Orange=species added Camp Tour: 4 Day 9 - December 11th; Canopy Camp am & Pan American Hwy. pm Day 10 - December 12th; El Salto Road am & Pan American Hwy. pm Day 11 - December 13th; El Salto Road am & Lagos Blancas pm There are a few trip photos at end! Day 12 - December 14th; Aligondi am & Sansom pm Day 13 - December 15th; San Francisco Reserve am 3rd 4th 5th 6th 7th 8th 9th 10th 11th 12th 13th 14th 15th PAPILIONIDAE SWALLOWTAILS Papilioninae Swallowtails & Cattlehearts Neographium agesilaus Short-lined Kite-Swallowtail X Battus polydamas Polydamas Swallowtail X Battus ingenuus Dyar's Swallowtail X Parides childrenae childrenae Green-celled Cattleheart X X X X X X Parides sesostris tarquinius Emerald-patched Cattleheart X X X XCC X X Heraclides anchisiades Ruby-spotted Swallowtail X Heraclides