North Andean Origin and Diversification of the Largest Ithomiine Butterfly Genus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proceedings of the United States National Museum
Proceedings of the United States National Museum SMITHSONIAN INSTITUTION • WASHINGTON, D.C. Volume 111 1960 Number 3429 A REVISION OF THE GENUS OGCODES LATREILLE WITH PARTICULAR REFERENCE TO SPECIES OF THE WESTERN HEMISPHERE By Evert I. Schlinger^ Introduction The cosmopolitan Ogcodes is the largest genus of the acrocerid or spider-parasite family. As the most highly evolved member of the subfamily Acrocerinae, I place it in the same general line of develop- ment as Holops Philippi, Villalus Cole, Thersitomyia Hunter, and a new South African genus.^ Ogcodes is most closely associated with the latter two genera . The Ogcodes species have never been treated from a world point of view, and this probably accounts for the considerable confusion that exists in the literature. However, several large regional works have been published that were found useful: Cole (1919, Nearctic), Brunetti (192G, miscellaneous species of the world, mostly from Africa and Australia), Pleske (1930, Palaearctic), Sack (1936. Palaearctic), and Sabrosky (1944, 1948, Nearctic). Up to this time 97 specific names have been applied to species and subspecies of this genus. Of these, 19 were considered synonyms, hence 78 species were assumed valid. With the description of 14 new species and the addition of one new name while finding onl}^ five new synonyms, 1 Department of Biological Control, University of California, Riverside, Calif. • This new genus, along with other new species and genera, is being described in forthcoming papers by the author. 227 228 PROCEEDINGS OF THE NATIONAL MUSEUM vol. m we find there are now 88 world species and subspecies. Thus, the total number of known forms is increased by 16 percent. -

INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a Synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a Historical Sketch
ZOOLOGÍA-TAXONOMÍA www.unal.edu.co/icn/publicaciones/caldasia.htm Caldasia 31(2):407-440. 2009 HACIA UNA SÍNTESIS DE LOS PAPILIONOIDEA (INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a historical sketch JOSÉ LUIS SALINAS-GUTIÉRREZ El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] CLAUDIO MÉNDEZ Escuela de Biología, Universidad de San Carlos, Ciudad Universitaria, Campus Central USAC, Zona 12. Guatemala, Guatemala. [email protected] MERCEDES BARRIOS Centro de Estudios Conservacionistas (CECON), Universidad de San Carlos, Avenida La Reforma 0-53, Zona 10, Guatemala, Guatemala. [email protected] CARMEN POZO El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] JORGE LLORENTE-BOUSQUETS Museo de Zoología, Facultad de Ciencias, UNAM. Apartado Postal 70-399, México D.F. 04510; México. [email protected]. Autor responsable. RESUMEN La riqueza biológica de Mesoamérica es enorme. Dentro de esta gran área geográfi ca se encuentran algunos de los ecosistemas más diversos del planeta (selvas tropicales), así como varios de los principales centros de endemismo en el mundo (bosques nublados). Países como Guatemala, en esta gran área biogeográfi ca, tiene grandes zonas de bosque húmedo tropical y bosque mesófi lo, por esta razón es muy importante para analizar la diversidad en la región. Lamentablemente, la fauna de mariposas de Guatemala es poco conocida y por lo tanto, es necesario llevar a cabo un estudio y análisis de la composición y la diversidad de las mariposas (Lepidoptera: Papilionoidea) en Guatemala. -

Butterflies (Lepidoptera: Papilionoidea) in a Coastal Plain Area in the State of Paraná, Brazil
62 TROP. LEPID. RES., 26(2): 62-67, 2016 LEVISKI ET AL.: Butterflies in Paraná Butterflies (Lepidoptera: Papilionoidea) in a coastal plain area in the state of Paraná, Brazil Gabriela Lourenço Leviski¹*, Luziany Queiroz-Santos¹, Ricardo Russo Siewert¹, Lucy Mila Garcia Salik¹, Mirna Martins Casagrande¹ and Olaf Hermann Hendrik Mielke¹ ¹ Laboratório de Estudos de Lepidoptera Neotropical, Departamento de Zoologia, Universidade Federal do Paraná, Caixa Postal 19.020, 81.531-980, Curitiba, Paraná, Brazil Corresponding author: E-mail: [email protected]٭ Abstract: The coastal plain environments of southern Brazil are neglected and poorly represented in Conservation Units. In view of the importance of sampling these areas, the present study conducted the first butterfly inventory of a coastal area in the state of Paraná. Samples were taken in the Floresta Estadual do Palmito, from February 2014 through January 2015, using insect nets and traps for fruit-feeding butterfly species. A total of 200 species were recorded, in the families Hesperiidae (77), Nymphalidae (73), Riodinidae (20), Lycaenidae (19), Pieridae (7) and Papilionidae (4). Particularly notable records included the rare and vulnerable Pseudotinea hemis (Schaus, 1927), representing the lowest elevation record for this species, and Temenis huebneri korallion Fruhstorfer, 1912, a new record for Paraná. These results reinforce the need to direct sampling efforts to poorly inventoried areas, to increase knowledge of the distribution and occurrence patterns of butterflies in Brazil. Key words: Atlantic Forest, Biodiversity, conservation, inventory, species richness. INTRODUCTION the importance of inventories to knowledge of the fauna and its conservation, the present study inventoried the species of Faunal inventories are important for providing knowledge butterflies of the Floresta Estadual do Palmito. -

Evolutionary Consequences of Dioecy in Angiosperms: the Effects of Breeding System on Speciation and Extinction Rates
EVOLUTIONARY CONSEQUENCES OF DIOECY IN ANGIOSPERMS: THE EFFECTS OF BREEDING SYSTEM ON SPECIATION AND EXTINCTION RATES by JANA C. HEILBUTH B.Sc, Simon Fraser University, 1996 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE STUDIES (Department of Zoology) We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA July 2001 © Jana Heilbuth, 2001 Wednesday, April 25, 2001 UBC Special Collections - Thesis Authorisation Form Page: 1 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. The University of British Columbia Vancouver, Canada http://www.library.ubc.ca/spcoll/thesauth.html ABSTRACT Dioecy, the breeding system with male and female function on separate individuals, may affect the ability of a lineage to avoid extinction or speciate. Dioecy is a rare breeding system among the angiosperms (approximately 6% of all flowering plants) while hermaphroditism (having male and female function present within each flower) is predominant. Dioecious angiosperms may be rare because the transitions to dioecy have been recent or because dioecious angiosperms experience decreased diversification rates (speciation minus extinction) compared to plants with other breeding systems. -
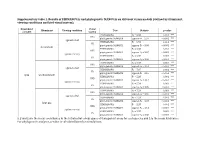
Supplementary Table 1. Results of Permanovas and Phylogenetic Manovas on Different Vision Models (Defined by Illuminant, Viewing Conditions and Bird Visual System)
Supplementary table 1. Results of PERMANOVAs and phylogenetic MANOVAs on different vision models (defined by illuminant, viewing conditions and bird visual system). Dependent Visual Illuminant Viewing condition Test Statistic p-value variable system PERMANOVA F9 = 6.88 0.001 *** UVS phylogenetic MANOVA approx-F9 = 2.97 < 0.001 *** against a leaf PERMANOVA F9 = 6.93 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.05 < 0.001 *** forest shade PERMANOVA F9 = 5.38 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** against the sky PERMANOVA F9 = 5.38 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.36 < 0.001 *** PERMANOVA F9 = 7.04 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.01 < 0.001 *** against a leaf PERMANOVA F9 = 7.07 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.10 < 0.001 *** xyzL woodland shade PERMANOVA F9 = 5.33 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.12 < 0.001 *** against the sky PERMANOVA F9 = 5.34 0.002 ** VS phylogenetic MANOVA approx-F9 = 3.39 < 0.001 *** PERMANOVA F9 = 7.24 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.00 < 0.001 *** against a leaf PERMANOVA F9 = 7.24 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** large gap PERMANOVA F9 = 5.37 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.14 < 0.001 *** against the sky PERMANOVA F9 = 5.37 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.38 < 0.001 *** x, y and z are the mean coordinates in the tetrahedral colour space of transparent areas for each species and L is the mean luminance. -

A Molecular Phylogeny of the Solanaceae
TAXON 57 (4) • November 2008: 1159–1181 Olmstead & al. • Molecular phylogeny of Solanaceae MOLECULAR PHYLOGENETICS A molecular phylogeny of the Solanaceae Richard G. Olmstead1*, Lynn Bohs2, Hala Abdel Migid1,3, Eugenio Santiago-Valentin1,4, Vicente F. Garcia1,5 & Sarah M. Collier1,6 1 Department of Biology, University of Washington, Seattle, Washington 98195, U.S.A. *olmstead@ u.washington.edu (author for correspondence) 2 Department of Biology, University of Utah, Salt Lake City, Utah 84112, U.S.A. 3 Present address: Botany Department, Faculty of Science, Mansoura University, Mansoura, Egypt 4 Present address: Jardin Botanico de Puerto Rico, Universidad de Puerto Rico, Apartado Postal 364984, San Juan 00936, Puerto Rico 5 Present address: Department of Integrative Biology, 3060 Valley Life Sciences Building, University of California, Berkeley, California 94720, U.S.A. 6 Present address: Department of Plant Breeding and Genetics, Cornell University, Ithaca, New York 14853, U.S.A. A phylogeny of Solanaceae is presented based on the chloroplast DNA regions ndhF and trnLF. With 89 genera and 190 species included, this represents a nearly comprehensive genus-level sampling and provides a framework phylogeny for the entire family that helps integrate many previously-published phylogenetic studies within So- lanaceae. The four genera comprising the family Goetzeaceae and the monotypic families Duckeodendraceae, Nolanaceae, and Sclerophylaceae, often recognized in traditional classifications, are shown to be included in Solanaceae. The current results corroborate previous studies that identify a monophyletic subfamily Solanoideae and the more inclusive “x = 12” clade, which includes Nicotiana and the Australian tribe Anthocercideae. These results also provide greater resolution among lineages within Solanoideae, confirming Jaltomata as sister to Solanum and identifying a clade comprised primarily of tribes Capsiceae (Capsicum and Lycianthes) and Physaleae. -

Elevation in Tropical Sky Islands As the Common Driver in Structuring Genes and Communities of Freshwater Organisms
Research Collection Journal Article Elevation in tropical sky islands as the common driver in structuring genes and communities of freshwater organisms Author(s): Gueuning, Morgan; Suchan, Tomasz; Rutschmann, Sereina; Gattolliat, Jean-Luc; Jamsari, Jamsari; Kamil, Al I.; Pitteloud, Camille; Buerki, Sven; Balke, Michael; Sartori, Michel; Alvarez, Nadir Publication Date: 2017 Permanent Link: https://doi.org/10.3929/ethz-b-000217957 Originally published in: Scientific Reports 7(1), http://doi.org/10.1038/s41598-017-16069-y Rights / License: Creative Commons Attribution 4.0 International This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library www.nature.com/scientificreports OPEN Elevation in tropical sky islands as the common driver in structuring genes and communities of Received: 30 January 2017 Accepted: 7 November 2017 freshwater organisms Published: xx xx xxxx Morgan Gueuning1,2, Tomasz Suchan1,12, Sereina Rutschmann1,3, Jean-Luc Gattolliat1,4, Jamsari Jamsari5, Al Ihsan Kamil5, Camille Pitteloud1,6,7, Sven Buerki8,9, Michael Balke10, Michel Sartori 1,4 & Nadir Alvarez 1,11 Tropical mountains are usually characterized by a vertically-arranged sequence of ecological belts, which, in contrast to temperate habitats, have remained relatively stable in space across the Quaternary. Such long-lasting patterning of habitats makes them ideal to test the role of environmental pressure in driving ecological and evolutionary processes. Using Sumatran freshwater mayfy communities, we test whether elevation, rather than other spatial factors (i.e. volcanoes, watersheds) structures both species within communities and genes within species. Based on the analysis of 31 mayfy (Ephemeroptera) communities and restriction-site-associated-DNA sequencing in the four most ubiquitous species, we found elevation as the major spatial component structuring both species and genes in the landscape. -

Rev Iss Web Mec 13773 25-22 5765..5784
Molecular Ecology (2016) 25, 5765–5784 doi: 10.1111/mec.13773 Into the Andes: multiple independent colonizations drive montane diversity in the Neotropical clearwing butterflies Godyridina NICOLAS CHAZOT,*† KEITH R. WILLMOTT,‡ FABIEN L. CONDAMINE,§¶ DONNA LISA DE-SILVA,* ANDREV.L.FREITAS,**GERARDOLAMAS,†† HELENE MORLON,‡‡ CARLOS E. GIRALDO,§§ CHRIS D. JIGGINS,¶¶ MATHIEU JORON,*** JAMES MALLET,††† SANDRA URIBE‡‡‡ and MARIANNE ELIAS* *Institut de Systematique, Evolution, Biodiversite, ISYEB – UMR 7205 – CNRS MNHN UPMC EPHE, Museum national d’Histoire naturelle, Sorbonne Universites, 57 rue Cuvier CP50, F-75005 Paris, France, †Department of Biology, University of Lund, 223 62 Lund, Sweden, ‡McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, Gainesville, FL 32611, USA, §CNRS, UMR 5554 Institut des Sciences de l’Evolution (Universite de Montpellier), Place Eugene Bataillon, 34095 Montpellier, France, ¶Department of Biological Sciences, University of Alberta, T6G 2E9 Edmonton, AB, Canada, **Departamento de Zoologia and Museu de Zoologia, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, S~ao Paulo, Brazil, ††Museo de Historia Natural, Universidad Nacional de San Marcos, Lima, Peru, ‡‡IBENS, Ecole Normale Superieure, UMR 8197 CNRS, Paris, France, §§Grupo de Investigacion de Sanidad Vegetal, Universidad Catolica de Oriente, Rionegro, Antioquia, Colombia, ¶¶Department of Zoology, University of Cambridge, Cambridge, UK, ***Centre d’Ecologie Fonctionnelle et Evolutive, CEFE, UMR 5175 CNRS – EPHE – Universite de Montpellier – Universite Paul Valery Montpellier, 34293 Montpellier 5, France, †††Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138, USA, ‡‡‡Universidad Nacional de Colombia, sede Medellın, Medellın, Colombia Abstract Understanding why species richness peaks along the Andes is a fundamental question in the study of Neotropical biodiversity. -

Molecular Phylogenetics of the Neotropical Butterfly Subtribe Oleriina
Molecular Phylogenetics and Evolution 55 (2010) 1032–1041 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Molecular phylogenetics of the neotropical butterfly subtribe Oleriina (Nymphalidae: Danainae: Ithomiini) Donna Lisa de-Silva a,*, Julia J. Day a, Marianne Elias b,c, Keith Willmott d, Alaine Whinnett a, James Mallet a a Department of Genetics, Evolution and Environment, University College London, Wolfson House, 4 Stephenson Way, London NW1 2HE, UK b Imperial College London, Silwood Park, Buckhurst Road, Ascot, Berkshire SL5 7PY, UK c CNRS, UMR 7205, Muséum National d’Histoire Naturelle, 45 Rue Buffon, CP50, 75005 Paris, France d McGuire Center for Lepidoptera, Florida Museum of Natural History, University of Florida, P.O. Box 112710, Gainesville, FL 32611-2710, USA article info abstract Article history: The Oleriina is one of the most speciose subtribes of the neotropical nymphalid butterfly tribe Ithomiini. Received 9 September 2009 They are widely distributed across the Andes and Amazonian lowlands and like other ithomiines they are Revised 22 December 2009 involved in complex mimicry rings. This subtribe is of particular interest because it contains the most Accepted 9 January 2010 diverse ithomiine genus, Oleria, as well as two genera, Megoleria and Hyposcada, that feed on hostplants Available online 15 January 2010 not utilized elsewhere in the tribe. Here we present the first comprehensive species-level phylogeny for the Oleriina, representing 83% of recognised species in the group, and based on 6698 bp from eight mito- Keywords: chondrial (mt) and nuclear (nc) genes. Topologies are largely congruent for ncDNA and the concatenated Lepidoptera dataset and the genera Oleria, Hyposcada and Megoleria are recovered and well-supported, although Speciation Phylogeny strongly discordant genealogy between mtDNA and ncDNA suggest possible introgression among Hypos- Hybridization cada and Megoleria. -

Why Continue with Floristic Checklists in Mexico
Botanical Sciences 97 (4): 741-753. 2019 Received: February 15, 2019, accepted: July 15, 2019 DOI: 10.17129/botsci.2174 On line first: December 17, 2019 Taxonomy and Floristics/Taxonomía y Florística Why continue with floristic checklists in Mexico? The case of the Tacaná- Boquerón Priority Terrestrial Region, in the Mexican State of Chiapas ¿Por qué continuar realizando listados florísticos en México? El caso de la Región Terrestre Prioritaria Tacaná-Boquerón, Chiapas Rubén Martínez-Camilo1*, Nayely Martínez-Meléndez2, Manuel Martínez-Meléndez3,4, Miguel Ángel Pérez- Farrera3, and Derio Antonio Jiménez-López1 1 Centro del Cambio Global y La Sustentabilidad A.C., Villahermosa, Tabasco, México. 2 El Colegio de la Frontera Sur, San Cristóbal de Las Casas, Chiapas, México. 3 Herbario Eizi Matuda; Laboratorio de Ecología Evolutiva, Instituto de Ciencias Biológicas, Universidad de Ciencias y Artes de Chiapas, Tuxtla Gutiérrez, Chiapas, México. 4 Eizia A.C., Tuxtla Gutiérrez, Chiapas, México. * Corresponding author: [email protected] Abstract Background: Some regions of Mexico have been relatively well explored floristically and estimates of the vascular plant richness they contain have been obtained. However, there are still regions that require effort to obtain the most appropriate lists of flora possible that consider both systemization of the information and that benefit from recent botanical explorations. Questions: What is the species richness of vascular plants in the Tacaná-Boquerón Priority Terrestrial Region? What proportion of the species are endemic or included in risk categories? Study sites and dates: Tacaná-Boquerón Priority Terrestrial Region, Chiapas State, Mexico. This region is on the Guatemala border and covers an area of 57,400 ha. -

Morphology of Male and Female Genitalia Across Acraea (Nymphalidae) Butterfly Species with and Without a Mating Plug
Morphology of male and female genitalia across Acraea (Nymphalidae) butterfly species with and without a mating plug Shannon L. Summers, Akito Y. Kawahara, & Ana P. S. Carvalho University of Florida Faculty Mentor, Akito Kawahara, Florida Museum of Natural History Abstract Male mating plugs have been used in many species to prevent female re-mating and sperm competition. One of the most extreme examples of a mating plug is the sphragis, which is a large, complex and externalized plug found only in butterflies. This structure is found in many species in the genus Acraea (Nymphalidae) and provides an opportunity for investigation of the effects of the sphragis on the morphology of the genitalia. This study aims to understand morphological interspecific variation in the genitalia of Acraea butterflies. Using museum collection specimens, abdomen dissections were conducted on 19 species of Acraea: 9 sphragis-bearing and 10 non-sphragis-bearing species. Genitalia were photographed and then measured using ImageJ software. Some distinguishing morphological features in the females were found. The most obvious difference is the larger and more externalized copulatory opening in sphragis-bearing species, with varying degrees of external projections. Females of the sphragis-bearing species also tend to have a shorter ductus (the structure that connects the copulatory opening with the sperm storage organ) than those without the sphragis. These differences may be due to a sexually antagonistic coevolution between the males and females, where the females evolve larger copulatory openings that are more difficult to plug, while males attempt to prevent re-mating with larger plugs (sphragis). Keywords: Lepidoptera, mate conflict, mating plug, sexual coevolution Introduction Sexual conflict occurs when there are different selection pressures on males and females. -

Illustrations of New Species of Exotic Butterflies
^^3 ILLUSTRATIONS OP NEW SPECIES OP EXOTIC BUTTERFLIES WILLIAM C. HBWITSON. lit SELECTED CHIEFLY FEOM HIS OWN COLLECTION. VOL. V. JOHN VAN VOORST, LONDON. 1872—1876. LONDON : PRINTED BY WOODFALL AND KINDER, . MILFORD LANE, STRAND, W.O. PREFACE. It is with regret, not however unmixed with satisfaction, tliat I come to the close of a work which has been to me a twenty-five years' labour of love. With regret that age and failing health forbid me to commence another volume. With satisfaction when I remember the great kindness which I have experienced personally from aU lepidopterists during its progress, and the very favourable reception it has met with from aE, and especially from those whose position as naturalists gives value to their opinions. I have many times during the progress of the book expressed my grati- tude for the kindness and liberality which I have met with from Dr. Boisduval, not only in giving me free access to his collections, but in allowing me during his absence to select from them and bring home with me aU that I wished to figure, feeling, as I do, the difiiculty I should myself experience in being parted from any of my treasures. To the generous encouragement met with from Mr. Wilson Saunders, especially at its commencement, the work owes much of its success. I am fully aware of and regret many errors, but have endeavoured to atone for them as much as possible by myself pointing them out and correcting them. It has always been my study to make the work useful rather than attractive.