Phylogenetic Revision of Eryphanis Boisduval, with a Description of a New Species from Ecuador (Lepidoptera, Nymphalidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

North Andean Origin and Diversification of the Largest Ithomiine Butterfly Genus
North Andean origin and diversification of the largest ithomiine butterfly genus The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Lisa De-Silva, D., L. L. Mota, N. Chazot, R. Mallarino, K. L. Silva- Brandão, L. M. G. Piñerez, A. V. Freitas, et al. 2017. “North Andean origin and diversification of the largest ithomiine butterfly genus.” Scientific Reports 7 (1): 45966. doi:10.1038/srep45966. http:// dx.doi.org/10.1038/srep45966. Published Version doi:10.1038/srep45966 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:32630680 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA www.nature.com/scientificreports OPEN North Andean origin and diversification of the largest ithomiine butterfly genus Received: 31 October 2016 Donna Lisa De-Silva1, Luísa L. Mota2, Nicolas Chazot1,3, Ricardo Mallarino4, Karina L. Silva- Accepted: 22 February 2017 Brandão5, Luz Miryam Gómez Piñerez6,7, André V.L. Freitas2, Gerardo Lamas8, Published: 07 April 2017 Mathieu Joron9, James Mallet4, Carlos E. Giraldo6,10, Sandra Uribe6, Tiina Särkinen11, Sandra Knapp12, Chris D. Jiggins13, Keith R. Willmott14 & Marianne Elias1 The Neotropics harbour the most diverse flora and fauna on Earth. The Andes are a major centre of diversification and source of diversity for adjacent areas in plants and vertebrates, but studies on insects remain scarce, even though they constitute the largest fraction of terrestrial biodiversity. -

Phylogenetic Relationships and Historical Biogeography of Tribes and Genera in the Subfamily Nymphalinae (Lepidoptera: Nymphalidae)
Blackwell Science, LtdOxford, UKBIJBiological Journal of the Linnean Society 0024-4066The Linnean Society of London, 2005? 2005 862 227251 Original Article PHYLOGENY OF NYMPHALINAE N. WAHLBERG ET AL Biological Journal of the Linnean Society, 2005, 86, 227–251. With 5 figures . Phylogenetic relationships and historical biogeography of tribes and genera in the subfamily Nymphalinae (Lepidoptera: Nymphalidae) NIKLAS WAHLBERG1*, ANDREW V. Z. BROWER2 and SÖREN NYLIN1 1Department of Zoology, Stockholm University, S-106 91 Stockholm, Sweden 2Department of Zoology, Oregon State University, Corvallis, Oregon 97331–2907, USA Received 10 January 2004; accepted for publication 12 November 2004 We infer for the first time the phylogenetic relationships of genera and tribes in the ecologically and evolutionarily well-studied subfamily Nymphalinae using DNA sequence data from three genes: 1450 bp of cytochrome oxidase subunit I (COI) (in the mitochondrial genome), 1077 bp of elongation factor 1-alpha (EF1-a) and 400–403 bp of wing- less (both in the nuclear genome). We explore the influence of each gene region on the support given to each node of the most parsimonious tree derived from a combined analysis of all three genes using Partitioned Bremer Support. We also explore the influence of assuming equal weights for all characters in the combined analysis by investigating the stability of clades to different transition/transversion weighting schemes. We find many strongly supported and stable clades in the Nymphalinae. We are also able to identify ‘rogue’ -

Plantas Alimenticias De 19 Especies De Mariposas Diurnas (Lepidoptera) En Loreto, Perú
Revista peruana de biología 24(1): 035 - 042 (2017) Plantas alimenticias de 19 especies de mariposasISSN-L 1561-0837 diurnas doi: http://dx.doi.org/10.15381/rpb.v24i1.13109 Facultad de Ciencias Biológicas UNMSM TRABAJOS ORIGINALES Plantas alimenticias de 19 especies de mariposas diurnas (Lepidoptera) en Loreto, Perú Food Plants of 19 butterflies species (Lepidoptera) from Loreto, Peru Joel Vásquez Bardales *1,2, Ricardo Zárate Gómez 1,3, Percy Huiñapi Canaquiri 1,2, Julio Pinedo Jiménez 4, Juan José Ramírez Hernández 5, Gerardo Lamas 5, Pedro Vela García 1,2 1 Instituto de Investigaciones de la Amazonía Peruana (IIAP). Av. A. Quiñones km 2.5, Iquitos, Loreto, Perú 2 Programa de Investigación en Biodiversidad Amazónica (PIBA). 3 Programa de Investigación en Cambio Climático, Desarrollo Territorial y Ambiente (PROTERRA). 4 Universidad Nacional de la Amazonia Peruana, Facultad de Agronomía. Calle Pevas s/n, Iquitos Perú 5 Universidad Nacional Mayor de San Marcos, Museo de Historia Natural. Av. Arenales 1256, Jesús María, Lima, Perú. *Autor para correspondencia Email Joel Vásquez Bardales: [email protected] Email Ricardo Zárate Gómez: [email protected] Email Percy Huiñapi Canaquiri: [email protected] Email Julio Pinedo Jiménez: [email protected] Email Juan José Ramírez Hernández: [email protected] Email Gerardo Lamas: [email protected] Email Pedro Vela García: [email protected] Resumen El presente trabajo informa sobre las plantas alimenticias utilizadas por 19 especies de mariposas diurnas (Lepidoptera) que ocurren en el Centro de Investigaciones Allpahuayo-Mishana y la Comunidad Campesina de San Rafael, Loreto, Perú. Se reportan 23 especies y 1 híbrido de angiospermas empleadas por las mariposas investigadas. -

A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1967 A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Ross, Gary Noel, "A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico." (1967). LSU Historical Dissertations and Theses. 1315. https://digitalcommons.lsu.edu/gradschool_disstheses/1315 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 67-14,010 ROSS, Gary Noel, 1940- A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO. Louisiana State University and Agricultural and Mechanical CoUege, Ph.D., 1967 Entomology University Microfilms, Inc., Ann Arbor, Michigan A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO A D issertation Submitted to the Graduate Faculty of the Louisiana State University and A gricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Entomology by Gary Noel Ross M.S., Louisiana State University, 196*+ May, 1967 FRONTISPIECE Section of the south wall of the crater of Volcan Santa Marta. May 1965, 5,100 feet. ACKNOWLEDGMENTS Many persons have contributed to and assisted me in the prep aration of this dissertation and I wish to express my sincerest ap preciation to them all. -

Nymphalidae, Brassolinae) from Panama, with Remarks on Larval Food Plants for the Subfamily
Journal of the Lepidopterists' Society 5,3 (4), 1999, 142- 152 EARLY STAGES OF CALICO ILLIONEUS AND C. lDOMENEUS (NYMPHALIDAE, BRASSOLINAE) FROM PANAMA, WITH REMARKS ON LARVAL FOOD PLANTS FOR THE SUBFAMILY. CARLA M. PENZ Department of Invertebrate Zoology, Milwaukee Public Museum, 800 West Wells Street, Milwaukee, Wisconsin 53233, USA , and Curso de P6s-Gradua9ao em Biocicncias, Pontiffcia Universidade Cat61ica do Rio Grande do SuI, Av. Ipiranga 6681, FOlto Alegre, RS 90619-900, BRAZIL ANNETTE AIELLO Smithsonian Tropical Research Institute, Apdo. 2072, Balboa, Ancon, HEPUBLIC OF PANAMA AND ROBERT B. SRYGLEY Smithsonian Tropical Research Institute, Apdo. 2072, Balboa, Ancon, REPUBLIC OF PANAMA, and Department of Zoology, University of Oxford, South Parks Road, Oxford, OX13PS, ENGLAND ABSTRACT, Here we describe the complete life cycle of Galigo illioneus oberon Butler and the mature larva and pupa of C. idomeneus (L.). The mature larva and pupa of each species are illustrated. We also provide a compilation of host records for members of the Brassolinae and briefly address the interaction between these butterflies and their larval food plants, Additional key words: Central America, host records, monocotyledonous plants, larval food plants. The nymphalid subfamily Brassolinae includes METHODS Neotropical species of large body size and crepuscular habits, both as caterpillars and adults (Harrison 1963, Between 25 May and .31 December, 1994 we Casagrande 1979, DeVries 1987, Slygley 1994). Larvae searched for ovipositing female butterflies along generally consume large quantities of plant material to Pipeline Road, Soberania National Park, Panama, mo reach maturity, a behavior that may be related as much tivated by a study on Caligo mating behavior (Srygley to the low nutrient content of their larval food plants & Penz 1999). -

INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a Synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a Historical Sketch
ZOOLOGÍA-TAXONOMÍA www.unal.edu.co/icn/publicaciones/caldasia.htm Caldasia 31(2):407-440. 2009 HACIA UNA SÍNTESIS DE LOS PAPILIONOIDEA (INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a historical sketch JOSÉ LUIS SALINAS-GUTIÉRREZ El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] CLAUDIO MÉNDEZ Escuela de Biología, Universidad de San Carlos, Ciudad Universitaria, Campus Central USAC, Zona 12. Guatemala, Guatemala. [email protected] MERCEDES BARRIOS Centro de Estudios Conservacionistas (CECON), Universidad de San Carlos, Avenida La Reforma 0-53, Zona 10, Guatemala, Guatemala. [email protected] CARMEN POZO El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] JORGE LLORENTE-BOUSQUETS Museo de Zoología, Facultad de Ciencias, UNAM. Apartado Postal 70-399, México D.F. 04510; México. [email protected]. Autor responsable. RESUMEN La riqueza biológica de Mesoamérica es enorme. Dentro de esta gran área geográfi ca se encuentran algunos de los ecosistemas más diversos del planeta (selvas tropicales), así como varios de los principales centros de endemismo en el mundo (bosques nublados). Países como Guatemala, en esta gran área biogeográfi ca, tiene grandes zonas de bosque húmedo tropical y bosque mesófi lo, por esta razón es muy importante para analizar la diversidad en la región. Lamentablemente, la fauna de mariposas de Guatemala es poco conocida y por lo tanto, es necesario llevar a cabo un estudio y análisis de la composición y la diversidad de las mariposas (Lepidoptera: Papilionoidea) en Guatemala. -

Butterflies (Lepidoptera: Papilionoidea) in a Coastal Plain Area in the State of Paraná, Brazil
62 TROP. LEPID. RES., 26(2): 62-67, 2016 LEVISKI ET AL.: Butterflies in Paraná Butterflies (Lepidoptera: Papilionoidea) in a coastal plain area in the state of Paraná, Brazil Gabriela Lourenço Leviski¹*, Luziany Queiroz-Santos¹, Ricardo Russo Siewert¹, Lucy Mila Garcia Salik¹, Mirna Martins Casagrande¹ and Olaf Hermann Hendrik Mielke¹ ¹ Laboratório de Estudos de Lepidoptera Neotropical, Departamento de Zoologia, Universidade Federal do Paraná, Caixa Postal 19.020, 81.531-980, Curitiba, Paraná, Brazil Corresponding author: E-mail: [email protected]٭ Abstract: The coastal plain environments of southern Brazil are neglected and poorly represented in Conservation Units. In view of the importance of sampling these areas, the present study conducted the first butterfly inventory of a coastal area in the state of Paraná. Samples were taken in the Floresta Estadual do Palmito, from February 2014 through January 2015, using insect nets and traps for fruit-feeding butterfly species. A total of 200 species were recorded, in the families Hesperiidae (77), Nymphalidae (73), Riodinidae (20), Lycaenidae (19), Pieridae (7) and Papilionidae (4). Particularly notable records included the rare and vulnerable Pseudotinea hemis (Schaus, 1927), representing the lowest elevation record for this species, and Temenis huebneri korallion Fruhstorfer, 1912, a new record for Paraná. These results reinforce the need to direct sampling efforts to poorly inventoried areas, to increase knowledge of the distribution and occurrence patterns of butterflies in Brazil. Key words: Atlantic Forest, Biodiversity, conservation, inventory, species richness. INTRODUCTION the importance of inventories to knowledge of the fauna and its conservation, the present study inventoried the species of Faunal inventories are important for providing knowledge butterflies of the Floresta Estadual do Palmito. -

A Distinctive New Species of Cloud Forest Euptychiina (Lepidoptera: Nymphalidae: Satyrinae) from Ecuador and Peru
WILLMOTT ET AL.: New species of Erichthodes TROP. LEPID. RES., 28(1): 39-45, 2018 39 A distinctive new species of cloud forest Euptychiina (Lepidoptera: Nymphalidae: Satyrinae) from Ecuador and Peru Keith R. Willmott1, Gerardo Lamas2, James Radford3, Mario A. Marín4, Shinichi Nakahara1, Marianne Espeland5, Lei Xiao1, and Jason P. W. Hall6 1. McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, Gainesville, FL, USA: [email protected] 2. Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Peru. 3. Cambridge, UK. 4. Departamento de Biologia Animal and Museu de Zoologia, Instituto de Biologia, Universidade Estadual de Campinas, Rua Monteiro Lobato, 255 - Cidade Universitária Zeferino Vaz - Barão Geraldo, 13083-862, Campinas, São Paulo, Brazil. 5. Arthropoda Department, Zoological Research Museum Alexander Koenig, Adenauer Allee 160, 53113 Bonn, Germany. 6. Department of Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA Date of issue online: 13 July 2018 Zoobank Registered: urn:lsid:zoobank.org:pub:F4A0F8EB-600F-4973-9D52-DDA7E27C3EF8 Electronic copies (ISSN 2575-9256) in PDF format at: http://journals.fcla.edu/troplep; https://zenodo.org; archived by the Institutional Repository at the University of Florida (IR@UF), http://ufdc.ufl.edu/ufir;DOI : 10.5281/zenodo.1309677 © The author(s). This is an open access article distributed under the Creative Commons license CC BY-NC 4.0 (https://creativecommons.org/ licenses/by-nc/4.0/). Abstract: A new species of Euptychiina, Erichthodes eremita Lamas, Willmott & Radford, n. sp., is described and illustrated. DNA sequence data suggest that the new species is sister to a species currently placed in Erichthodes Forster, 1964, although ongoing revision of the generic taxonomy of the subtribe might result in the reclassification of both of these species in future. -

Combining Taxonomic and Functional Approaches to Unravel the Spatial Distribution of an Amazonian Butterfly Community
Environmental Entomology Advance Access published December 7, 2015 Environmental Entomology, 2015, 1–9 doi: 10.1093/ee/nvv183 Community and Ecosystem Ecology Research article Combining Taxonomic and Functional Approaches to Unravel the Spatial Distribution of an Amazonian Butterfly Community Marlon B. Grac¸a,1,2,3 Jose´W. Morais,1 Elizabeth Franklin,1,2 Pedro A. C. L. Pequeno,1,2 Jorge L. P. Souza,1,2 and Anderson Saldanha Bueno,1,4 1Biodiversity Coordination, National Institute for Amazonian Research, INPA, Manaus, Brazil ([email protected]; [email protected]; [email protected]; [email protected]; [email protected]; [email protected]), 2Center for Integrated Studies of Amazonian Biodiversity, CENBAM, Manaus, Brazil, 3Corresponding author, e-mail: marlon_lgp@hotmail. com, and 4Campus Ju´lio de Castilhos, Farroupilha Federal Institute of Education, Science and Technology, Brazil ([email protected]) Received 24 August 2015; Accepted 10 November 2015 Abstract This study investigated the spatial distribution of an Amazonian fruit-feeding butterfly assemblage by linking spe- cies taxonomic and functional approaches. We hypothesized that: 1) vegetation richness (i.e., resources) and abun- dance of insectivorous birds (i.e., predators) should drive changes in butterfly taxonomic composition, 2) larval diet breadth should decrease with increase of plant species richness, 3) small-sized adults should be favored by higher abundance of birds, and 4) communities with eyespot markings should be able to exploit areas with higher predation pressure. Fruit-feeding butterflies were sampled with bait traps and insect nets across 25 km2 of an Amazonian ombrophilous forest in Brazil. We measured larval diet breadth, adult body size, and wing marking of all butterflies. -

Check List Lists of Species Check List 11(6): 1813, 15 December 2015 Doi: ISSN 1809-127X © 2015 Check List and Authors
11 6 1813 the journal of biodiversity data 15 December 2015 Check List LISTS OF SPECIES Check List 11(6): 1813, 15 December 2015 doi: http://dx.doi.org/10.15560/11.6.1813 ISSN 1809-127X © 2015 Check List and Authors Butterflies (Lepidoptera: Papilionoidea and Hesperioidea) of the Banhado dos Pachecos Wildlife Refuge, Uruguayan Savanna Ecoregion, Rio Grande do Sul state, Brazil Andressa Caporale1, Liana Bertoldi Moreno1, Nicolás Oliveira Mega1, 2*, Helena Piccoli Romanowski1, 2 1 Graduate Program in Animal Biology. Federal University of Rio Grande do Sul. Av. Bento Gonçalves 9500/43435. CEP 91504-970. Porto Alegre, RS, Brazil 2 Department of Zoology. Federal University of Rio Grande do Sul. Av. Bento Gonçalves 9500/43435. CEP 91504-970. Porto Alegre, RS, Brazil * Corresponding author. E-mail: [email protected] Abstract: The Pampa is a biome shared by Argentina, the Pampa presents high biodiversity and is home to Brazil, and Uruguay. Despite its high biodiversity, little is very characteristic flora and fauna. Estimates indicate known about the invertebrate fauna. The few inventories the presence of approximately 3,000 plant species, over done so far were conducted outside protected areas, 100 mammal species, and almost 500 bird species (MMA which could result in underestimated real biodiversity. 2007). Thus, species inventories from protected areas should Despite its high biodiversity, little is known about be done to serve as reference for conservation. Here invertebrate diversity in the Pampa. Considering that we survey the butterflies occurring in the Banhado dos only recent studies focus on non-pest insects, some Pachecos Wildlife Refuge, Uruguayan Savanna, Brazil. -
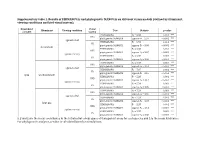
Supplementary Table 1. Results of Permanovas and Phylogenetic Manovas on Different Vision Models (Defined by Illuminant, Viewing Conditions and Bird Visual System)
Supplementary table 1. Results of PERMANOVAs and phylogenetic MANOVAs on different vision models (defined by illuminant, viewing conditions and bird visual system). Dependent Visual Illuminant Viewing condition Test Statistic p-value variable system PERMANOVA F9 = 6.88 0.001 *** UVS phylogenetic MANOVA approx-F9 = 2.97 < 0.001 *** against a leaf PERMANOVA F9 = 6.93 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.05 < 0.001 *** forest shade PERMANOVA F9 = 5.38 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** against the sky PERMANOVA F9 = 5.38 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.36 < 0.001 *** PERMANOVA F9 = 7.04 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.01 < 0.001 *** against a leaf PERMANOVA F9 = 7.07 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.10 < 0.001 *** xyzL woodland shade PERMANOVA F9 = 5.33 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.12 < 0.001 *** against the sky PERMANOVA F9 = 5.34 0.002 ** VS phylogenetic MANOVA approx-F9 = 3.39 < 0.001 *** PERMANOVA F9 = 7.24 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.00 < 0.001 *** against a leaf PERMANOVA F9 = 7.24 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** large gap PERMANOVA F9 = 5.37 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.14 < 0.001 *** against the sky PERMANOVA F9 = 5.37 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.38 < 0.001 *** x, y and z are the mean coordinates in the tetrahedral colour space of transparent areas for each species and L is the mean luminance. -

Elevation in Tropical Sky Islands As the Common Driver in Structuring Genes and Communities of Freshwater Organisms
Research Collection Journal Article Elevation in tropical sky islands as the common driver in structuring genes and communities of freshwater organisms Author(s): Gueuning, Morgan; Suchan, Tomasz; Rutschmann, Sereina; Gattolliat, Jean-Luc; Jamsari, Jamsari; Kamil, Al I.; Pitteloud, Camille; Buerki, Sven; Balke, Michael; Sartori, Michel; Alvarez, Nadir Publication Date: 2017 Permanent Link: https://doi.org/10.3929/ethz-b-000217957 Originally published in: Scientific Reports 7(1), http://doi.org/10.1038/s41598-017-16069-y Rights / License: Creative Commons Attribution 4.0 International This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library www.nature.com/scientificreports OPEN Elevation in tropical sky islands as the common driver in structuring genes and communities of Received: 30 January 2017 Accepted: 7 November 2017 freshwater organisms Published: xx xx xxxx Morgan Gueuning1,2, Tomasz Suchan1,12, Sereina Rutschmann1,3, Jean-Luc Gattolliat1,4, Jamsari Jamsari5, Al Ihsan Kamil5, Camille Pitteloud1,6,7, Sven Buerki8,9, Michael Balke10, Michel Sartori 1,4 & Nadir Alvarez 1,11 Tropical mountains are usually characterized by a vertically-arranged sequence of ecological belts, which, in contrast to temperate habitats, have remained relatively stable in space across the Quaternary. Such long-lasting patterning of habitats makes them ideal to test the role of environmental pressure in driving ecological and evolutionary processes. Using Sumatran freshwater mayfy communities, we test whether elevation, rather than other spatial factors (i.e. volcanoes, watersheds) structures both species within communities and genes within species. Based on the analysis of 31 mayfy (Ephemeroptera) communities and restriction-site-associated-DNA sequencing in the four most ubiquitous species, we found elevation as the major spatial component structuring both species and genes in the landscape.